Molecular mechanism of multivesicular body biogenesis by ESCRT complexes - PubMed (original) (raw)
Molecular mechanism of multivesicular body biogenesis by ESCRT complexes
Thomas Wollert et al. Nature. 2010.
Abstract
When internalized receptors and other cargo are destined for lysosomal degradation, they are ubiquitinated and sorted by the endosomal sorting complex required for transport (ESCRT) complexes 0, I, II and III into multivesicular bodies. Multivesicular bodies are formed when cargo-rich patches of the limiting membrane of endosomes bud inwards by an unknown mechanism and are then cleaved to yield cargo-bearing intralumenal vesicles. The biogenesis of multivesicular bodies was reconstituted and visualized using giant unilamellar vesicles, fluorescent ESCRT-0, -I, -II and -III complexes, and a membrane-tethered fluorescent ubiquitin fusion as a model cargo. Here we show that ESCRT-0 forms domains of clustered cargo but does not deform membranes. ESCRT-I and ESCRT-II in combination deform the membrane into buds, in which cargo is confined. ESCRT-I and ESCRT-II localize to the bud necks, and recruit ESCRT-0-ubiquitin domains to the buds. ESCRT-III subunits localize to the bud neck and efficiently cleave the buds to form intralumenal vesicles. Intralumenal vesicles produced in this reaction contain the model cargo but are devoid of ESCRTs. The observations explain how the ESCRTs direct membrane budding and scission from the cytoplasmic side of the bud without being consumed in the reaction.
Figures
Fig. 1
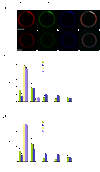
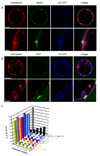
Model cargo clustering by ESCRT-0. a, ESCRT-0 induces the formation of Ub domains. (b) Ub mutant I44D is not clustered under the same conditions as in (a). Tagged Ub fusions were present at 65 nM. (c) Clusters of ESCRT-0 (15 nM, labeled with Alexa 488) can form in the presence of Ub I44D, but the size and number of clusters is reduced. The presence of 15 nM ESCRT-I and II has little effect on the clustering. (d) Ub-CFP clustering by ESCRT-0 is prevented in the I44D mutant. Error bars in (c) and (d) were calculated from the standard deviation of n = 3 separate experiments. Scale bar = 10 µm.
Fig. 2
ESCRT-I and –II-induce membrane buds and confine cargo in them. Labeled ESCRT-I and unlabeled ESCRT-II (a) and vice versa (b) induce buds, as summarized (c). (d, e) Fluorescence recovery after photobleaching (FRAP) experiments of model cargo confined in buds. Arrows indicate buds in which Ub-GFP does not significantly recover on the time scale of the experiment. Error bars in were calculated from the standard deviation of n = 3 separate experiments in part (c) and for n = 10 different buds each selected from a different GUV in (e). Scale bar = 10 µm.
Fig. 3
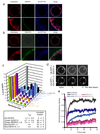
ESCRT-I and II localize to the necks of membrane buds. Imaging was carried out in the presence of 15 nM unlabeled Vps20, which binds to ESCRT-II and augments ESCRT-I and II occupancy at the neck. ESCRT-I and –II were present at 15 nM each in both experiments. Labeled ESCRT-I in the presence of unlabeled ESCRT-II (a) and labeled ESCRT-II in the presence of unlabeled ESCRT-I (b) induce membrane buds and co-localize with their necks. Scale bar = 10 µm and 2 µm (insets).
Fig. 4
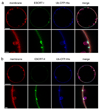
ESCRT-0 Ub domains co-localize with ESCRT-I-II membrane buds. (a) Wildtype yeast ESCRT-0 (15 nM, Alexa 488-labelled) in the presence of 65 nM His6-Ub-CFP colocalizes with buds induced by 15 nM each unlabelled ESCRT-I and –II. The second row shows a close-up of an individual cluster-bud pair. (b) ESCRT-0 mutated in three P(S/T)XP motifs required for ESCRT-I binding does not colocalizes with membrane buds. (c) Results for 70 GUVs are summarized in histograms. Error bars in were calculated from the standard deviation of n = 3 separate experiments. Scale bar = 10 µm (upper panels showing entire GUV) and 2 µm (inset).
Fig. 5
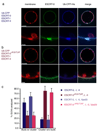
ESCRT-III localizes to bud necks for membrane scission. 15 nM each of unlabeled ESCRT-0, ESCRT-I, and ESCRT-II, and 65 nM of His-Ub-GFP were present in both experiments. a,Vps20 (15 nM, labeled with Alexa 488) is localized to bud necks but does not sever the necks. b, Snf7 (15 nM, labeled with Alexa 488) localizes to bud necks when Vps20 (15 nM, unlabeled) is present. (c) The dependence of ILV production on Snf7 is indicated by the histogram of observations on 100 GUVs. Error bars in were calculated from the standard deviation of n = 3 separate experiments. Scale bar = 10 µm (upper panels showing entire GUV) and 2 µm (inset).
Fig. 6
Molecular mechanism of MVB biogenesis. a, ESCRT-0 self-assembles and clusters cargo. b, The ESCRT-I and –II contain multiple membrane binding sites separated by a rigid stalk in ESCRT-I and a rigid Y-structure in ESCRT-II that could prop open membrane necks. c, ESCRT-II recruits Vps20 to the neck, which in turn recruits Snf7, the main engine for neck scission. d, Following scission, cargo is internalized in ILVs while ESCRT-III remains on the outside of the limiting membrane until it is recycled by Vps4.
Comment in
- Endocytosis: division of labour between ESCRTs.
David R. David R. Nat Rev Mol Cell Biol. 2010 May;11(5):314. doi: 10.1038/nrm2888. Nat Rev Mol Cell Biol. 2010. PMID: 20422742
Similar articles
- Reconstituting multivesicular body biogenesis with purified components.
Wollert T. Wollert T. Methods Cell Biol. 2012;108:73-92. doi: 10.1016/B978-0-12-386487-1.00004-3. Methods Cell Biol. 2012. PMID: 22325598 - Binding to any ESCRT can mediate ubiquitin-independent cargo sorting.
Mageswaran SK, Dixon MG, Curtiss M, Keener JP, Babst M. Mageswaran SK, et al. Traffic. 2014 Feb;15(2):212-29. doi: 10.1111/tra.12135. Epub 2013 Nov 14. Traffic. 2014. PMID: 24148098 Free PMC article. - The endosomal sorting complex ESCRT-II mediates the assembly and architecture of ESCRT-III helices.
Henne WM, Buchkovich NJ, Zhao Y, Emr SD. Henne WM, et al. Cell. 2012 Oct 12;151(2):356-71. doi: 10.1016/j.cell.2012.08.039. Cell. 2012. PMID: 23063125 - Membrane budding and scission by the ESCRT machinery: it's all in the neck.
Hurley JH, Hanson PI. Hurley JH, et al. Nat Rev Mol Cell Biol. 2010 Aug;11(8):556-66. doi: 10.1038/nrm2937. Epub 2010 Jun 30. Nat Rev Mol Cell Biol. 2010. PMID: 20588296 Free PMC article. Review. - ESCRT-dependent cargo sorting at multivesicular endosomes.
Frankel EB, Audhya A. Frankel EB, et al. Semin Cell Dev Biol. 2018 Feb;74:4-10. doi: 10.1016/j.semcdb.2017.08.020. Epub 2017 Aug 8. Semin Cell Dev Biol. 2018. PMID: 28797838 Free PMC article. Review.
Cited by
- Crystal structure of the Thr316Ala mutant of a yeast JAMM deubiquitinase: implication of active-site loop dynamics in catalysis.
Shrestha R, Das C. Shrestha R, et al. Acta Crystallogr F Struct Biol Commun. 2021 Jun 1;77(Pt 6):163-170. doi: 10.1107/S2053230X21005124. Epub 2021 May 24. Acta Crystallogr F Struct Biol Commun. 2021. PMID: 34100774 Free PMC article. - Microglial microvesicle secretion and intercellular signaling.
Turola E, Furlan R, Bianco F, Matteoli M, Verderio C. Turola E, et al. Front Physiol. 2012 May 22;3:149. doi: 10.3389/fphys.2012.00149. eCollection 2012. Front Physiol. 2012. PMID: 22661954 Free PMC article. - Free energy calculations for membrane morphological transformations and insights to physical biology and oncology.
Parihar K, Ko SH, Bradley R, Taylor P, Ramakrishnan N, Baumgart T, Guo W, Weaver VM, Janmey PA, Radhakrishnan R. Parihar K, et al. Methods Enzymol. 2024;701:359-386. doi: 10.1016/bs.mie.2024.03.028. Epub 2024 Apr 14. Methods Enzymol. 2024. PMID: 39025576 Free PMC article. - Extracellular Vesicles in Amyotrophic Lateral Sclerosis.
McCluskey G, Morrison KE, Donaghy C, Rene F, Duddy W, Duguez S. McCluskey G, et al. Life (Basel). 2022 Dec 31;13(1):121. doi: 10.3390/life13010121. Life (Basel). 2022. PMID: 36676070 Free PMC article. Review. - Exosomes derived from cancerous and non-cancerous cells regulate the anti-tumor response in the tumor microenvironment.
Bae S, Brumbaugh J, Bonavida B. Bae S, et al. Genes Cancer. 2018 Mar;9(3-4):87-100. doi: 10.18632/genesandcancer.172. Genes Cancer. 2018. PMID: 30108680 Free PMC article. Review.
References
- Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell Biol. 2004;5:317–323. - PubMed
- Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. - PubMed
- Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell. 2002;3:283–289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases