3D 23Na MRI of human skeletal muscle at 7 Tesla: initial experience - PubMed (original) (raw)
3D 23Na MRI of human skeletal muscle at 7 Tesla: initial experience
Gregory Chang et al. Eur Radiol. 2010 Aug.
Abstract
Objective: To evaluate healthy skeletal muscle pre- and post-exercise via 7 T (23)Na MRI and muscle proton T(2) mapping, and to evaluate diabetic muscle pre- and post-exercise via 7 T (23)Na MRI.
Methods: The calves of seven healthy subjects underwent imaging pre- and post-exercise via 7 T (23)Na MRI (3D fast low angle shot, TR/TE = 80 ms/0.160 ms, 4 mm x 4 mm x 4 mm) and 1 week later by (1)H MRI (multiple spin-echo sequence, TR/TE = 3,000 ms/15-90 ms). Four type 2 diabetics also participated in the (23)Na MRI protocol. Pre- and post-exercise sodium signal intensity (SI) and proton T(2) relaxation values were measured/calculated for soleus (S), gastrocnemius (G), and a control, tibialis anterior (TA). Two-tailed t tests were performed.
Results: In S/G in healthy subjects post-exercise, sodium SI increased 8-13% (p < 0.03), then decreased (t(1/2) = 22 min), and (1)H T(2) values increased 12-17% (p < 0.03), then decreased (t(1/2 )= 12-15 min). In TA, no significant changes in sodium SI or (1)H T(2) values were seen (-2.4 to 1%, p > 0.17). In S/G in diabetics, sodium SI increased 10-11% (p < 0.04), then decreased (t(1/2) = 27-37 min) without significant change in the TA SI (-3.6%, p = 0.066).
Conclusion: It is feasible to evaluate skeletal muscle via 3D (23)Na MRI at 7 T. Post-exercise muscle (1)H T(2) values return to baseline more rapidly than sodium SI. Diabetics may demonstrate delayed muscle sodium SI recovery compared with healthy subjects.
Figures
Fig. 1
Plot of sodium signal intensity vs. phantom NaCl concentration (100–300 mM/L) with background signal intensity plotted as 0 mM/L NaCl. There is a linear relationship between sodium MR signal and NaCl concentration. The results of three experiments performed on different days are plotted
Fig. 2
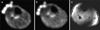
a Resting sodium MR image (TR=80 ms, TE=0.160 ms, 4 mm × 4 mm × 4 mm) from the calf of a healthy volunteer. The NaCl phantoms can be seen at the anterior aspect of the leg. b Immediate post-exercise sodium MR image from the same volunteer, showing subtle increase in sodium signal intensity within the posterior compartment muscles. c Corresponding proton 3D fast low angle shot (FLASH) MR image (TR=20 ms, TE=5 ms, 0.195 mm × 0.195 mm, 1-mm slice thickness) from the same volunteer, which was performed after the sodium MRI to delineate muscle anatomy. TA tibialis anterior, S soleus, G gastrocnemius. Coil inhomogeneity artefact is seen at the anterior and posterior aspects of the leg
Fig. 3
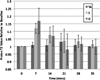
Bar graph demonstrating the time course of recovery for sodium signal intensity after exercise in healthy subjects. Immediately after exercise, sodium signal intensity increased in S (8±4%, _p_=0.028) and G (13±8%, _p_=0.028), but not in the dorsiflexor control muscle TA (0.55%, _p_=0.92). Sodium signal intensity then decreased to near baseline in an exponential fashion with a _t_1/2 of approximately 22 min for both S and G
Fig. 4
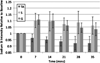
Bar graph demonstrating changes in proton T2 relaxation values before and after exercise. Baseline T2 relaxation values for tibialis anterior (TA), soleus (S) and gastrocnemius (G) were 33.9± 3.78 ms, 36.8±4.2 ms and 36.7±4.67 ms, respectively. Immediately after exercise, T2 relaxation values increased in S (12±3%, _p_=0.01) and G (17±8%, _p_=0.029), but not in TA (1±2%, _p_=0.18). T2 relaxation values in S and G then decreased in a logarithmic fashion with half-lives (_t_1/2) of approximately 15 and 12 min, respectively
Fig. 5
Bar graph demonstrating the time course of recovery for sodium signal intensity after exercise in diabetics. Immediately after exercise, sodium signal intensity increased in S (10±6%, _p_=0.034) and G (11±4%, _p_=0.009), but not in TA (−3.6±4%, _p_=0.066). Over time, sodium SI in S and G gradually decreased with half-lives (_t_1/2) of approximately 37 and 27 min, respectively
Similar articles
- 3 Tesla (23)Na magnetic resonance imaging during aerobic and anaerobic exercise.
Hammon M, Grossmann S, Linz P, Kopp C, Dahlmann A, Janka R, Cavallaro A, Uder M, Titze J. Hammon M, et al. Acad Radiol. 2015 Sep;22(9):1181-90. doi: 10.1016/j.acra.2015.06.005. Epub 2015 Jul 4. Acad Radiol. 2015. PMID: 26152501 - Sodium and quantitative hydrogen parameter changes in muscle tissue after eccentric exercise and in delayed-onset muscle soreness assessed with magnetic resonance imaging.
Höger SA, Gast LV, Marty B, Hotfiel T, Bickelhaupt S, Uder M, Heiss R, Nagel AM. Höger SA, et al. NMR Biomed. 2023 Feb;36(2):e4840. doi: 10.1002/nbm.4840. Epub 2022 Nov 5. NMR Biomed. 2023. PMID: 36196511 - Sodium quantification in skeletal muscle: comparison between Cartesian gradient-echo and radial ultra-short echo time 23Na MRI techniques.
Gerhalter T, Schilling F, Zeitouni N, Linz P, Baudin PY, Kannenkeril D, Kopp C, Dahlmann A, Schmieder R, Uder M, Nagel AM, Gast LV. Gerhalter T, et al. Eur Radiol Exp. 2024 May 22;8(1):61. doi: 10.1186/s41747-024-00461-1. Eur Radiol Exp. 2024. PMID: 38773044 Free PMC article. - Biochemical and physiological MR imaging of skeletal muscle at 7 tesla and above.
Chang G, Wang L, Cárdenas-Blanco A, Schweitzer ME, Recht MP, Regatte RR. Chang G, et al. Semin Musculoskelet Radiol. 2010 Jun;14(2):269-78. doi: 10.1055/s-0030-1253167. Epub 2010 May 18. Semin Musculoskelet Radiol. 2010. PMID: 20486034 Free PMC article. Review. - Sodium MRI in human heart: a review.
Bottomley PA. Bottomley PA. NMR Biomed. 2016 Feb;29(2):187-96. doi: 10.1002/nbm.3265. Epub 2015 Feb 12. NMR Biomed. 2016. PMID: 25683054 Free PMC article. Review.
Cited by
- Biomedical applications of sodium MRI in vivo.
Madelin G, Regatte RR. Madelin G, et al. J Magn Reson Imaging. 2013 Sep;38(3):511-29. doi: 10.1002/jmri.24168. Epub 2013 May 30. J Magn Reson Imaging. 2013. PMID: 23722972 Free PMC article. Review. - Recent advances in medical physics.
Kalender WA, Quick HH. Kalender WA, et al. Eur Radiol. 2011 Mar;21(3):501-4. doi: 10.1007/s00330-010-2027-9. Epub 2010 Dec 5. Eur Radiol. 2011. PMID: 21132304 Review. - Review. The Agfa Mayneord lecture: MRI of short and ultrashort T₂ and T₂* components of tissues, fluids and materials using clinical systems.
Bydder GM. Bydder GM. Br J Radiol. 2011 Dec;84(1008):1067-82. doi: 10.1259/bjr/74368403. Br J Radiol. 2011. PMID: 22101579 Free PMC article. - A century of exercise physiology: effects of muscle contraction and exercise on skeletal muscle Na+,K+-ATPase, Na+ and K+ ions, and on plasma K+ concentration-historical developments.
McKenna MJ, Renaud JM, Ørtenblad N, Overgaard K. McKenna MJ, et al. Eur J Appl Physiol. 2024 Mar;124(3):681-751. doi: 10.1007/s00421-023-05335-9. Epub 2024 Jan 11. Eur J Appl Physiol. 2024. PMID: 38206444 Free PMC article. Review. - Permanent muscular sodium overload and persistent muscle edema in Duchenne muscular dystrophy: a possible contributor of progressive muscle degeneration.
Weber MA, Nagel AM, Wolf MB, Jurkat-Rott K, Kauczor HU, Semmler W, Lehmann-Horn F. Weber MA, et al. J Neurol. 2012 Nov;259(11):2385-92. doi: 10.1007/s00415-012-6512-8. Epub 2012 Apr 28. J Neurol. 2012. PMID: 22544297
References
- Fleckenstein JL, Canby RC, Parkey RW, Preshock RM. Acute effects of exercise on MR imaging of skeletal muscle in normal volunteers. AJR Am J Roentgenol. 1988;151:231–237. - PubMed
- Fleckenstein JL, Bertocci LA, Nunnally RL, Parkey RW, Peshock RM. Exercise-enhanced MR imaging of variation in forearm muscle anatomy and use: importance in MR spectros-copy. Am J Roentgenol. 1989;153:693–698. - PubMed
- Patten C, Meyer RA, Fleckenstein JL. T2 mapping of muscle. Semin Musculoskelet Radiol. 2003;7:297–305. - PubMed
- Meyer RA, Prior BM. Functional magnetic resonance imaging of muscle. Exerc Sport Sci Rev. 2000;28:89–92. - PubMed
- Damon BM, Gregory CD, Hall KL, et al. Intracellular acidification and volume increases explain R2 decreases in exercising muscle. Magn Reson Med. 2002;47:14–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical