Epiblast/germ line hypothesis of cancer development revisited: lesson from the presence of Oct-4+ cells in adult tissues - PubMed (original) (raw)
Review
Epiblast/germ line hypothesis of cancer development revisited: lesson from the presence of Oct-4+ cells in adult tissues
Mariusz Z Ratajczak et al. Stem Cell Rev Rep. 2010 Jun.
Abstract
The morphology of several tumors mimics developmentally early tissues; tumors often express early developmental markers characteristic for the germ line lineage. Recently, our group identified a population of very small stem cells (SCs) in murine bone marrow (BM) and other adult organs that express several markers characteristic for epiblast/germ line-derived SCs. We named these rare cells "Very Small Embryonic/Epiblast-like Stem Cells (VSELs)." We hypothesized that these cells that express both epiblast and germ line markers are deposited during early gastrulation in developing tissues and organs and play an important role in the turnover of tissue-committed (TC) SCs. To support this, we envision that the germ line is not only the origin of SCs, but also remains as a scaffold or back-up for the SC compartment in adult life. Furthermore, we noticed that VSELs are protected from uncontrolled proliferation and teratoma formation by a unique DNA methylation pattern in some developmentally crucial imprinted genes, which show hypomethylation or erasure of imprints in paternally methylated genes and hypermethylation of imprints in the maternally methylated. In pathological situations, however, we hypothesize that VSELs could be involved in the development of several malignancies. Therefore, potential involvement of VSELs in cancerogenesis could support century-old concepts of embryonic rest- or germ line-origin hypotheses of cancer development. However, we are aware that this working hypothesis requires further direct experimental confirmation.
Figures
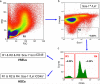
Fig. 1
BM preparation and gating strategy for sorting of murine BM-derived VSELs by FACS. Panel a Gating strategy for VSEL isolation by FACS: Agranular, small events ranging from 2 μm to 10 μm are included in gate R1 after comparison with six differently sized bead particles with standard diameters of 1, 2, 4, 6, 10, and 15 μm; cells are visualized by dot plot showing forward scatter (FSC) vs. side scatter (SSC) signals, which are related to the size and granularity/complexity of the cell, respectively. Panel b Cells from region R1 are further analyzed for Sca-1 and Lin expression and only Sca-1+/Lin− events are included into region R2. Panel c Population from region R2 is subsequently distinguished based on CD45 marker expression into CD45− and CD45+ subpopulations visualized on histogram. CD45−/Lin−/Sca-1+ cells (VSELs) are sorted as objects enclosed in a logical gate including regions R1, R2, and R3, while CD45+/Lin−/Sca-1+ cells (HSCs) are from a gate including regions R1, R2, and R4. Percentages show the average content of each cellular subpopulation in total BM nucleated cells
Fig. 2
The overall hypothesis presented in this review of the developmental deposition of Oct-4+ epiblast/germ line-derived VSELs in adult tissues. It is known that the germ line carries the genome (nuclear and mitochondrial DNA) from one generation to the next. Somatic SCs “bud out” from the germ line during ontogenesis and form soma, which help germ line-derived gametes fulfill this mission. The germ potential is established in the fertilized oocyte (zygote) during fertilization and subsequently retained in the morula, ICM of the blastocyst, epiblast, PGCs, and mature GCs (gonocytes–oocytes and sperm). The first cells that bud out from the germ lineage are trophoectodermal cells that give rise to the placenta. Subsequently, we envision that during gastrulation, the epiblast is a source of PSCs for all three germ layers (meso-, ecto-, and endoderm) as well as PGCs. We hypothesize that at this stage, some epiblast-derived SCs could be deposited as Oct-4+ PSCs in peripheral tissues and organs (red circles). In addition, some migrating PGCs could deviate from their major migratory route to the genital ridges and be deposited as well. Furthermore, we hypothesize that epiblast/germ line-derived SCs (VSELs) deposited in the developing tissues undergo erasure of their somatic imprint similarly to PGCs. This “erasure of methylation of somatic imprinted genes” protects the developing organism from the unwanted possibility of teratoma formation, but at the same time restrains their pluripotentiality
Fig. 3
Reprogramming of somatic imprints control the pluripotentiality and quiescence of VSELs. Panel a During development, PGCs and VSELs undergo similar epigenetic reprogramming of somatic imprinted genes; however, they also retain expression of some pluripotent genes (e.g., Oct-4). In particular, the erasure of somatic imprints in these cells is responsible for preventing them from aberrant teratoma formation, but at the same time restrains their pluripotentiality. In contrast, differentiated somatic cells lose their pluripotency by turning off the transcription of pluripotent genes through stable DNA methylation of their promoters (e.g, Oct-4). However, they retain the somatic pattern of the genomic imprint. Thus, somatic cells may be dedifferentiated to PSCs by expression of pluripotent genes (_blue box_—iPSC protocol). In contrast, PGCs that express pluripotential genes, but erase the somatic imprint, may become pluripotent EGCs by proper remethylation of some of erased imprinted genes (_green box_—EGC protocol). We hypothesize that similar modulation of parent-of-origin-specific reprogramming of somatic imprints in VSELs that enforces their quiescent status in tissues may “unleash” their pluripotentiality and reverse them to fully pluripotent status (_dark blue box_—VSEL protocol). Panel b Bisulfite sequencing results of DNA methylation of indicated imprinted loci in VSELs and HSCs. Mean values for the percentage of methylated CpG sites are shown as the mean±S.D. from at least three independent experiments. Dashed red line indicates the somatic imprinting pattern (50%). Panel c Relative quantification of the expression level of maternally expressed/proliferation-repressing imprinted genes (H19, p57KIP2,and Igf2R, blue box) and paternally expressed/proliferation-promoting genes (Igf2 and Rasgrf1, red box) in VSELs and HSCs. The relative expression level is represented as the fold difference to the value of BM mononuclear cells (BMMNCs) and shown as the mean from at least four independent experiments. VSELs, HSCs, and BMMNCs were separated by FACS from 4 week-old mice
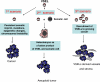
Fig. 4
Three scenarios postulated in this review for involvement of VSELs in cancerogenesis. 1st scenario: We envision that this particular scenario plays a significant role in the pathogenesis of pediatric tumors. Accordingly, VSELs may give rise to cancer (stem) cells (dark irregular cell) if the genomic imprinting is not erased or the VSEL acquires mutations and, as a result of this, potentially gives rise to teratomas/teratocarcinomas or sarcomas (e.g., rhabdomyosarcoma, neuroblastoma, nephroblastoma), respectively. 2nd scenario: VSELs may fuse with somatic cells and give rise to heterokaryons (blue cells with nucleus). Tetraploid heterokaryons undergo subsequent selection and give rise to aneuploid cancer (stem) cells. We envision that this scenario in particular plays a more important role in the pathogenesis of tumors developing on the basis of chronic inflammation/tissue injury in adult patients. 3rd scenario: VSELs may indirectly contribute to tumorogenesis by providing vasculature and stroma cells for the growing tumor tissue
Similar articles
- Molecular characterization of isolated from murine adult tissues very small embryonic/epiblast like stem cells (VSELs).
Shin DM, Liu R, Klich I, Ratajczak J, Kucia M, Ratajczak MZ. Shin DM, et al. Mol Cells. 2010 Jun;29(6):533-8. doi: 10.1007/s10059-010-0081-4. Epub 2010 Jun 4. Mol Cells. 2010. PMID: 20526817 Review. - Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4(+) very small embryonic-like stem cells.
Shin DM, Zuba-Surma EK, Wu W, Ratajczak J, Wysoczynski M, Ratajczak MZ, Kucia M. Shin DM, et al. Leukemia. 2009 Nov;23(11):2042-51. doi: 10.1038/leu.2009.153. Epub 2009 Jul 30. Leukemia. 2009. PMID: 19641521 Free PMC article. - Identification of very small embryonic/epiblast-like stem cells (VSELs) circulating in peripheral blood during organ/tissue injuries.
Ratajczak MZ, Liu R, Marlicz W, Blogowski W, Starzynska T, Wojakowski W, Zuba-Surma E. Ratajczak MZ, et al. Methods Cell Biol. 2011;103:31-54. doi: 10.1016/B978-0-12-385493-3.00003-6. Methods Cell Biol. 2011. PMID: 21722799 Review. - Very small embryonic-like stem cells: characterization, developmental origin, and biological significance.
Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Ratajczak J, Kucia M. Ratajczak MZ, et al. Exp Hematol. 2008 Jun;36(6):742-51. doi: 10.1016/j.exphem.2008.03.010. Exp Hematol. 2008. PMID: 18474305 Free PMC article. Review.
Cited by
- Reprogramming of gastrointestinal cancer cells.
Dewi D, Ishii H, Haraguchi N, Nishikawa S, Kano Y, Fukusumi T, Ohta K, Miyazaki S, Ozaki M, Sakai D, Satoh T, Nagano H, Doki Y, Mori M. Dewi D, et al. Cancer Sci. 2012 Mar;103(3):393-9. doi: 10.1111/j.1349-7006.2011.02184.x. Epub 2012 Jan 17. Cancer Sci. 2012. PMID: 22151786 Free PMC article. Review. - Gonadotropin treatment augments postnatal oogenesis and primordial follicle assembly in adult mouse ovaries?
Bhartiya D, Sriraman K, Gunjal P, Modak H. Bhartiya D, et al. J Ovarian Res. 2012 Nov 7;5(1):32. doi: 10.1186/1757-2215-5-32. J Ovarian Res. 2012. PMID: 23134576 Free PMC article. - Specialty Grand Challenge-Assisted Reproduction.
Lunenfeld E. Lunenfeld E. Front Reprod Health. 2021 Jul 26;3:551499. doi: 10.3389/frph.2021.551499. eCollection 2021. Front Reprod Health. 2021. PMID: 36304062 Free PMC article. No abstract available. - Newer insights into premeiotic development of germ cells in adult human testis using Oct-4 as a stem cell marker.
Bhartiya D, Kasiviswanathan S, Unni SK, Pethe P, Dhabalia JV, Patwardhan S, Tongaonkar HB. Bhartiya D, et al. J Histochem Cytochem. 2010 Dec;58(12):1093-106. doi: 10.1369/jhc.2010.956870. Epub 2010 Aug 30. J Histochem Cytochem. 2010. PMID: 20805580 Free PMC article. - Pituitary sex hormones enhance the pro‑metastatic potential of human lung cancer cells by downregulating the intracellular expression of heme oxygenase‑1.
Abdelbaset-Ismail A, Pedziwiatr D, Schneider G, Niklinski J, Charkiewicz R, Moniuszko M, Kucia M, Ratajczak MZ. Abdelbaset-Ismail A, et al. Int J Oncol. 2017 Jan;50(1):317-328. doi: 10.3892/ijo.2016.3787. Epub 2016 Dec 2. Int J Oncol. 2017. PMID: 27922667 Free PMC article.
References
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. - PubMed
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. - PubMed
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Medicine. 1997;3:730–737. - PubMed
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
- Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20RR018733/RR/NCRR NIH HHS/United States
- R01 DK074720/DK/NIDDK NIH HHS/United States
- P20 RR018733/RR/NCRR NIH HHS/United States
- R01 CA106281-01/CA/NCI NIH HHS/United States
- R01 CA106281/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources