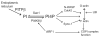Phosphatidylinositol- and phosphatidylcholine-transfer activity of PITPbeta is essential for COPI-mediated retrograde transport from the Golgi to the endoplasmic reticulum - PubMed (original) (raw)
. 2010 Apr 15;123(Pt 8):1262-73.
doi: 10.1242/jcs.061986. Epub 2010 Mar 23.
Affiliations
- PMID: 20332109
- PMCID: PMC2848114
- DOI: 10.1242/jcs.061986
Phosphatidylinositol- and phosphatidylcholine-transfer activity of PITPbeta is essential for COPI-mediated retrograde transport from the Golgi to the endoplasmic reticulum
Nicolas Carvou et al. J Cell Sci. 2010.
Abstract
Vesicles formed by the COPI complex function in retrograde transport from the Golgi to the endoplasmic reticulum (ER). Phosphatidylinositol transfer protein beta (PITPbeta), an essential protein that possesses phosphatidylinositol (PtdIns) and phosphatidylcholine (PtdCho) lipid transfer activity is known to localise to the Golgi and ER but its role in these membrane systems is not clear. To examine the function of PITPbeta at the Golgi-ER interface, RNA interference (RNAi) was used to knockdown PITPbeta protein expression in HeLa cells. Depletion of PITPbeta leads to a decrease in PtdIns(4)P levels, compaction of the Golgi complex and protection from brefeldin-A-mediated dispersal to the ER. Using specific transport assays, we show that anterograde traffic is unaffected but that KDEL-receptor-dependent retrograde traffic is inhibited. This phenotype can be rescued by expression of wild-type PITPbeta but not by mutants defective in docking, PtdIns transfer and PtdCho transfer. These data demonstrate that the PtdIns and PtdCho exchange activity of PITPbeta is essential for COPI-mediated retrograde transport from the Golgi to the ER.
Figures
Fig. 1.
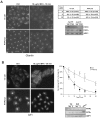
Depletion of PITPβ by RNAi causes Golgi compaction and deforms the nucleus. (A) Western blot showing knockdown of PITPβ or PITPα by siRNA specific for each protein. ARF1 was used as a loading control. Quantification of numerous blots indicates that the efficiency of knockdown after the second round of transfection (TF) was always greater than 90 % (supplementary material Fig. S1). (B) PITPα and/or PITPβ siRNA-treated cells were fixed 6 days after transfection, immunostained with a Golgi marker (giantin antibody), treated with DAPI and examined by microscopy. Representative images of control and PITP siRNA-treated cells are shown (×40 objective). (C) Golgi condensation in control and PITPβ siRNA-treated cells was quantified following immunostaining with the Golgi marker, GM130. Values are the percentage of cells with condensed Golgi from three experiments. (D) High magnification images with nuclear staining (×100 objective). (E) DAPI-stained nuclei of HeLa cells after siRNA transfection (×40 objective). Compared with control cells, which have regular oval shaped nuclei, those of PITPβ knockdown cells have atypical ‘kidney’ or ‘doughnut’ shapes. (F) Transmission EM images of control and PITPβ knockdown cells (see supplementary material Fig. S4 for a low magnification view of the juxtanuclear area).
Fig. 2.
Disassembly of the Golgi by BFA is delayed in cells depleted of PITPβ. HeLa cells transfected with control or PITPβ siRNA were treated with 10 μg/ml BFA at 37°C for the indicated times. (A) In PITPβ-knockdown cells, giantin staining shows a tight Golgi morphology and is protected from redistribution to the ER upon BFA treatment. Cells with conserved Golgi were quantified for both populations (right). (B) In the knockdown cells, ARF1 shows tight perinuclear staining and upon BFA treatment, ARF1 dissociation from the Golgi into the cytosol is retarded. The percentage of cells with ARF1 remaining at the Golgi was quantified in control and knockdown cells after BFA treatment (right). Ten random fields per time point were analysed (×40 objective). TF, transfection.
Fig. 3.
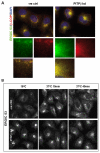
Depletion of PITPβ affects ERGIC-53 and β-COP localisation and inhibits the recycling pathway of ERGIC-53. (A) Control and PITPβ-knockdown cells were stained for ERGIC-53 and β-COPI. These proteins colocalise and in the PITPβ-knockdown cells the peripheral localisation of both ERGIC-53 and β-COPI is lost (see boxed area, and enlarged panels below). Instead both proteins show tight perinuclear staining. (B) HeLa cells transfected with control or PITPβ siRNA were incubated at 16°C for 3 hours to accumulate ERGIC-53 in the perinuclear Golgi region. The cells were then transferred to 37°C for the indicated times and redistribution of ERGIC-53 was monitored by immunostaining with an anti-ERGIC-53 antibody. ERGIC-53 is retained in the perinuclear region in PITPβ-knockdown cells after the temperature shift, whereas in control cells ERGIC-53 redistributes to the periphery of the cells.
Fig. 4.
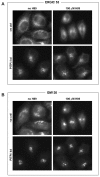
H89-induced relocalisation of ERGIC-53 to the ER is prevented in cells depleted of PITPβ. Control and PITPβ siRNA-treated cells were treated with 100 μM H89 for 30 minutes at 37°C and immunostained for (A) ERGIC-53 and (B) GM130.
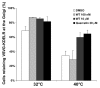
Fig. 5.
Knockdown of PITPβ inhibits COPI-mediated transport. (A) Retrograde transport of the chimaeric KDEL receptor (VSVGts045-KDEL-R) from the Golgi to the ER is delayed in PITPβ-knockdown cells. At the permissive temperature (32°C), the VSVGts045-KDEL-R is localised at the Golgi and after shifting to the non-permissive temperature (40°C), the receptor moves to the ER where it gets trapped. (B) The percentage of cells with VSVGts045-KDEL-R at the Golgi is quantified for control and PITPβ-knockdown cells. (C) Model demonstrating the trafficking step that is inhibited in PITPβ-knockdown cells. In control cells (top two panels), the construct cycles freely between the ER and the Golgi at the permissive temperature (32°C) and at steady state it is mainly at the cis-Golgi. Upon shift to the non-permissive temperature (40°C), the construct can move to the ER but gets trapped upon arrival at the ER. In PITPβ-knockdown cells (bottom two panels), the construct remains at the cis-Golgi even at the non-permissive temperature because of a blockade of retrograde trafficking.
Fig. 6.
PtdIns(4) P (PI4P) levels are reduced in PITPβ-knockdown cells. (A) HeLa cells treated with control and _PITPβ_-specific siRNA were grown in the presence of [3H]inositol for 3 days, and the lipids were extracted and analysed by TLC and phosphorimaged. (B) Quantification of the phosphoinositide levels from three independent experiments each carried out in triplicate. *P<0.05. PI, PtdIns; PIP, PtdIns(4)P; PIP2, PtdIns(4,5)_P_2.
Fig. 7.
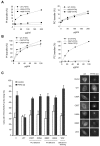
PtdIns and PtdCho transfer activity of PITPβ is required for rescue of retrograde transport from the Golgi to ER. (A) Mutation K60A and N89F causes loss of PtdIns (PI) but not PtdCho (PC) transfer. (B) Mutation of cysteine 95 to alanine or to threonine causes loss of PtdCho transfer but not PtdIns transfer. PtdIns and PtdCho transfer activity is expressed as a percentage of the maximum transfer observed with wild-type PITPβ. (C) Rescue of the retrograde traffic defect of VSVGts045-KDEL-R by wild-type PITPβ but not mutants deficient in PtdCho transfer (C95A and C95T), PtdIns transfer (N89F and K60A) and a mutant incapable of docking to the Golgi and ER membranes (WW203/204AA, respectively). Results were combined from three independent experiments (± s.d.). In each experiment, an average of 50 cells were counted per condition. (Right) Representative immunofluorescence images of VSVGts045-KDEL-R in control and PITPβ-knockdown cells rescued with either empty vector, wild-type PITPβ or PITPβ mutants.
Fig. 8.
Retrograde transport of VSVGts045-KDEL-R is blocked by inhibitors of type III PI 4-kinases. Cells were treated with the indicated concentrations of inhibitors for 2 hour and the percentage of cells retaining the chimaera at the Golgi was monitored. The number of cells counted per condition were 70-100 cells in each individual experiment and the results were compiled from three independent experiments (± s.e.m.).
Fig. 9.
Scheme illustrating how PITPβ participates in the retrograde Golgi to ER transport. PITPβ delivers PtdIns (PI) from the ER to the cis-Golgi, where it is phosphorylated to PtdIns(4)P (PI4P) by PI4KIIIβ. PtdIns(4)P directly or indirectly, via N-WASP and Cdc42 or via Syne-1 (nesprin1), regulates the balance of G- to F-actin; actin dynamics is required for COP1-complex function.
Similar articles
- PITPβ promotes COPI vesicle fission through lipid transfer and membrane contact formation.
Park K, Ju S, Choi H, Gao P, Bang G, Choi JH, Jang J, Morris AJ, Kang BH, Hsu VW, Park SY. Park K, et al. J Cell Biol. 2025 May 5;224(5):e202407166. doi: 10.1083/jcb.202407166. Epub 2025 Apr 11. J Cell Biol. 2025. PMID: 40214667 - The cytoplasmic tail of IBV spike mediates intracellular retention via interaction with COPI-coated vesicles in retrograde trafficking.
Liang R, Tian J, Liu K, Ma L, Yang R, Sun L, Zhao J, Zhao Y, Zhang G. Liang R, et al. J Virol. 2025 Feb 25;99(2):e0216424. doi: 10.1128/jvi.02164-24. Epub 2025 Jan 22. J Virol. 2025. PMID: 39840971 Free PMC article. - Coatomer complex I is required for the transport of SARS-CoV-2 progeny virions from the endoplasmic reticulum-Golgi intermediate compartment.
Hirabayashi A, Muramoto Y, Takenaga T, Tsunoda Y, Wakazaki M, Sato M, Fujita-Fujiharu Y, Nomura N, Yamauchi K, Onishi C, Nakano M, Toyooka K, Noda T. Hirabayashi A, et al. mBio. 2025 Jan 8;16(1):e0333124. doi: 10.1128/mbio.03331-24. Epub 2024 Nov 29. mBio. 2025. PMID: 39611845 Free PMC article. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - The 2023 Latin America report of the Lancet Countdown on health and climate change: the imperative for health-centred climate-resilient development.
Hartinger SM, Palmeiro-Silva YK, Llerena-Cayo C, Blanco-Villafuerte L, Escobar LE, Diaz A, Sarmiento JH, Lescano AG, Melo O, Rojas-Rueda D, Takahashi B, Callaghan M, Chesini F, Dasgupta S, Posse CG, Gouveia N, Martins de Carvalho A, Miranda-Chacón Z, Mohajeri N, Pantoja C, Robinson EJZ, Salas MF, Santiago R, Sauma E, Santos-Vega M, Scamman D, Sergeeva M, Souza de Camargo T, Sorensen C, Umaña JD, Yglesias-González M, Walawender M, Buss D, Romanello M. Hartinger SM, et al. Lancet Reg Health Am. 2024 Apr 23;33:100746. doi: 10.1016/j.lana.2024.100746. eCollection 2024 May. Lancet Reg Health Am. 2024. PMID: 38800647 Free PMC article. Review.
Cited by
- Phosphatidylinositol transfer proteins and instructive regulation of lipid kinase biology.
Grabon A, Khan D, Bankaitis VA. Grabon A, et al. Biochim Biophys Acta. 2015 Jun;1851(6):724-35. doi: 10.1016/j.bbalip.2014.12.011. Epub 2015 Jan 12. Biochim Biophys Acta. 2015. PMID: 25592381 Free PMC article. Review. - Non-vesicular phosphatidylinositol transfer plays critical roles in defining organelle lipid composition.
Kim YJ, Pemberton JG, Eisenreichova A, Mandal A, Koukalova A, Rohilla P, Sohn M, Konradi AW, Tang TT, Boura E, Balla T. Kim YJ, et al. EMBO J. 2024 May;43(10):2035-2061. doi: 10.1038/s44318-024-00096-3. Epub 2024 Apr 16. EMBO J. 2024. PMID: 38627600 Free PMC article. - Lipid Exchangers: Cellular Functions and Mechanistic Links With Phosphoinositide Metabolism.
Lipp NF, Ikhlef S, Milanini J, Drin G. Lipp NF, et al. Front Cell Dev Biol. 2020 Jul 21;8:663. doi: 10.3389/fcell.2020.00663. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32793602 Free PMC article. Review. - Homeostatic regulation of the PI(4,5)P2-Ca(2+) signaling system at ER-PM junctions.
Chang CL, Liou J. Chang CL, et al. Biochim Biophys Acta. 2016 Aug;1861(8 Pt B):862-873. doi: 10.1016/j.bbalip.2016.02.015. Epub 2016 Feb 24. Biochim Biophys Acta. 2016. PMID: 26924250 Free PMC article. Review. - Interdomain interactions regulate the localization of a lipid transfer protein at ER-PM contact sites.
Basak B, Krishnan H, Raghu P. Basak B, et al. Biol Open. 2021 Mar 18;10(3):bio057422. doi: 10.1242/bio.057422. Biol Open. 2021. PMID: 33597200 Free PMC article.
References
- Alb J. G., Jr, Cortese J. D., Phillips S. E., Albin R. L., Nagy T. R., Hamilton B. A., Bankaitis V. A. (2003). Mice lacking phosphatidylinositol transfer protein alpha exhibit spinocerebellar degeneration, intestinal and hepatic steatosis, and hypoglycemia. J. Biol. Chem. 278, 33501-33518 - PMC - PubMed
- Aridor M., Balch W. E. (2000). Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum. J. Biol. Chem. 275, 35673-35676 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials