CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials - PubMed (original) (raw)
Guideline
CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials
Kenneth F Schulz et al. BMJ. 2010.
Abstract
The CONSORT statement is used worldwide to improve the reporting of randomised controlled trials. Kenneth Schulz and colleagues describe the latest version, CONSORT 2010, which updates the reporting guideline based on new methodological evidence and accumulating experience
Conflict of interest statement
Competing interests: Uniform disclosure of potential conflicts of interest: all authors have completed the ICMJE unified competing interest form at www.icmje.org/coi\_disclosure.pdf (available from the corresponding author) and declare (1) DM received grants for this work from Johnson & Johnson, BMJ, and American Society for Clinical Oncology; KFS and DGA received support for travel to meetings for this work from Johnson & Johnson, BMJ, and American Society for Clinical Oncology; (2) KFS and DA had travel expenses reimbursed by the EQUATOR Network; KFS has received honoraria for delivering educational presentations for the American Board of Obstetrics and Gynecology Foundation for Excellence in Women’s Health Care, Ortho-McNeil Janssen Scientific Affairs, and the American College of Obstetrics and Gynecology; and has done consultancy for Wyeth. All authors also declare (3) no spouses, partners, or children with relationships with commercial entities that might have an interest in the submitted work; (4) no non-financial interests that may be relevant to the submitted work.
Figures
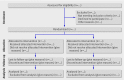
Flow diagram of the progress through the phases of a parallel randomised trial of two groups (that is, enrolment, intervention allocation, follow-up, and data analysis)
Comment in
- The new CONSORT statement.
Antes G. Antes G. BMJ. 2010 Mar 23;340:c1432. doi: 10.1136/bmj.c1432. BMJ. 2010. PMID: 20332507 No abstract available.
Similar articles
- CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials.
Schulz KF, Altman DG, Moher D; CONSORT Group. Schulz KF, et al. J Clin Epidemiol. 2010 Aug;63(8):834-40. doi: 10.1016/j.jclinepi.2010.02.005. Epub 2010 Mar 25. J Clin Epidemiol. 2010. PMID: 20346629 No abstract available. - CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials.
Schulz KF, Altman DG, Moher D; CONSORT Group. Schulz KF, et al. BMC Med. 2010 Mar 24;8:18. doi: 10.1186/1741-7015-8-18. BMC Med. 2010. PMID: 20334633 Free PMC article. - CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials.
Schulz KF, Altman DG, Moher D; CONSORT Group. Schulz KF, et al. Trials. 2010 Mar 24;11:32. doi: 10.1186/1745-6215-11-32. Trials. 2010. PMID: 20334632 Free PMC article. - CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials.
Schulz KF, Altman DG, Moher D; CONSORT Group. Schulz KF, et al. Int J Surg. 2011;9(8):672-7. doi: 10.1016/j.ijsu.2011.09.004. Epub 2011 Oct 13. Int J Surg. 2011. PMID: 22019563 No abstract available. - CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials.
Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG; CONSORT. Moher D, et al. Int J Surg. 2012;10(1):28-55. doi: 10.1016/j.ijsu.2011.10.001. Epub 2011 Oct 12. Int J Surg. 2012. PMID: 22036893 Review.
Cited by
- NSE and S100β as serum alarmins in predicting neurological outcomes after cardiac arrest.
Hu J, Ai M, Xie S, Qian Z, Zhang L, Huang L. Hu J, et al. Sci Rep. 2024 Oct 26;14(1):25539. doi: 10.1038/s41598-024-76979-6. Sci Rep. 2024. PMID: 39462073 Free PMC article. - The WE-RISE™ multi-domain intervention: a feasibility study for the potential reversal of cognitive frailty in Malaysian older persons of lower socioeconomic status.
Murukesu RR, Shahar S, Subramaniam P, Mohd Rasdi HF, Nur AM, Singh DKA. Murukesu RR, et al. BMC Geriatr. 2024 Oct 31;24(1):903. doi: 10.1186/s12877-024-05457-5. BMC Geriatr. 2024. PMID: 39482612 Free PMC article. Clinical Trial. - Effects of self-management interventions based on the COM-B model on peri-implant condition in older adults with periodontitis: a randomized controlled trial.
Hu M, Yu L, Cao Y, Ding Z, Ma H, Gao Y, Zhu F. Hu M, et al. BMC Oral Health. 2024 Oct 23;24(1):1267. doi: 10.1186/s12903-024-05064-1. BMC Oral Health. 2024. PMID: 39443915 Free PMC article. Clinical Trial. - A systematic review with attempted network meta-analysis of asthma therapy recommended for five to eighteen year olds in GINA steps three and four.
van der Mark LB, Lyklema PH, Geskus RB, Mohrs J, Bindels PJ, van Aalderen WM, Ter Riet G. van der Mark LB, et al. BMC Pulm Med. 2012 Oct 15;12:63. doi: 10.1186/1471-2466-12-63. BMC Pulm Med. 2012. PMID: 23067257 Free PMC article. Review. - Protocol for an 'efficient design' cluster randomised controlled trial to evaluate a complex intervention to improve antibiotic prescribing for CHIldren presenting to primary care with acute COugh and respiratory tract infection: the CHICO study.
Seume P, Bevan S, Young G, Ingram J, Clement C, Cabral C, Lucas PJ, Beech E, Taylor J, Horwood J, Dixon P, Gulliford MC, Francis N, Creavin ST, Lane A, Hay AD, Blair PS. Seume P, et al. BMJ Open. 2021 Mar 29;11(3):e041769. doi: 10.1136/bmjopen-2020-041769. BMJ Open. 2021. PMID: 33782018 Free PMC article.
References
- Chan AW, Altman DG. Epidemiology and reporting of randomised trials published in PubMed journals. Lancet 2005;365:1159-62. - PubMed
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637-9. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources