Mechanisms behind estrogen's beneficial effect on muscle strength in females - PubMed (original) (raw)
Review
Mechanisms behind estrogen's beneficial effect on muscle strength in females
Dawn A Lowe et al. Exerc Sport Sci Rev. 2010 Apr.
Abstract
Muscle weakness ensues when serum testosterone declines with age in men. Testosterone's female counterpart, estrogen, also has been implicated in age-related strength loss, but these results are less conclusive. Our working hypothesis is that estrogens do benefit muscle strength, and that the underlying mechanism involves estrogen receptors to improve muscle quality more so than quantity.
Figures
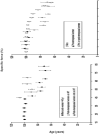
Figure 1
Relationship between specific muscle force of the adductor pollicis muscle and age for groups of subjects that did and did not experience loss of estrogens during the sixth decade of life. Within each graph, specific force is expressed as a percent of the mean for subjects aged 45 yr and younger. Top panel: Following menopause, women lose muscle strength with age at a greater rate than do men out until about the age of 70 yr. Bottom panel: Postmenopausal women who take an estrogen-based hormone therapy (HT) retain muscle strength to a greater extent than do women who do not take the therapy. Figure created from data originally published in (22).
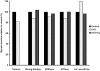
Figure 2
Forest plots of effect sizes from meta-analyses on studies that reported muscle strength normalized to muscle size (specific force) in subjects that were and were not estrogen deficient. Each square represents the effect size for that study with the size of the square equating to the weight of that study in the meta-analysis. The horizontal line through the square indicates the 95% confidence interval (CI) for that effect size. Within each plot, studies are arranged from lowest to highest effect sizes. Top panel: Results of five studies on postmenopausal women comparing muscle strength between women who were and were not on an estrogen-based hormone therapy. Bottom panel: Results of seven studies on rats or mice comparing specific force between those that were estradiol deficient via ovariectomy and those that were ovary intact or estradiol-replaced. Figure created from data originally published in (8); refer to this paper for references cited in the figure.
Figure 3
Decrements in the functions of myosin occur when muscle fibers or myofibrils are analyzed from rodents deficient in estrogens via ovariectomy (OVX). Conversely, when ovariectomized rodents are treated with estradiol (OVX+E2), myosin functions return to control levels. Tension is specific force generated by permeabilized fibers and is reflective of the overall ability of acto-myosin filaments to make force (data from (32)). The fraction of myosin in the strong-binding structural state during contraction directly reflects the force-generating capacity of myosin and active stiffness of intact muscle indirectly reflects myosin strongly bound to actin during contraction; both are affected by estrogen status (data from reference #16). Both myofibrillar adenosine triphosphate hydrolysis (ATPase) and control of calcium (Ca2+) are required for myosin to cycle between strong- and weak-binding states to actin and both are influenced by estrogens (data from (33)).
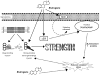
Figure 4
Schematic of how estrogens may benefit muscle strength. Ovariectomized mice display a reduction in muscle strength and this is attributed to a decrement in the fraction of strong-binding myosin during contraction. Conversely, treatment with estrogens increases strong-binding myosin and ultimately strength. Estrogen receptor (ER) content in muscle is responsive to circulating estrogens, and we hypothesize that ERs may initiate signaling cascades and/or regulate genes that result in an overall reduction in oxidative stress in fibers. Alternatively, estrogens may have some direct antioxidant affect. We speculate that reducing oxidative stress would preserve myosin structure-function, conferring a beneficial effect on strength. Solid arrows represent experimental evidence from our lab and others. Dashed arrows represent hypothesized mechanisms of estrogens’ actions in skeletal muscle. Abbreviations: adenosine triphosphate (ATP), inorganic phosphate (Pi), G protein-coupled receptor (Gper).
Similar articles
- Aging of the musculoskeletal system: How the loss of estrogen impacts muscle strength.
Collins BC, Laakkonen EK, Lowe DA. Collins BC, et al. Bone. 2019 Jun;123:137-144. doi: 10.1016/j.bone.2019.03.033. Epub 2019 Mar 28. Bone. 2019. PMID: 30930293 Free PMC article. Review. - Differential sensitivity of estrogen target tissues: implications for estrogen regulation of serum luteinizing hormone.
Kelner KL, Peck EJ Jr. Kelner KL, et al. J Neurosci Res. 1984;11(1):79-89. doi: 10.1002/jnr.490110109. J Neurosci Res. 1984. PMID: 6708135 - Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle.
Baltgalvis KA, Greising SM, Warren GL, Lowe DA. Baltgalvis KA, et al. PLoS One. 2010 Apr 13;5(4):e10164. doi: 10.1371/journal.pone.0010164. PLoS One. 2010. PMID: 20405008 Free PMC article. - Skeletal muscle and bone: effect of sex steroids and aging.
Brown M. Brown M. Adv Physiol Educ. 2008 Jun;32(2):120-6. doi: 10.1152/advan.90111.2008. Adv Physiol Educ. 2008. PMID: 18539850 - The nongenomic protective effects of estrogen on the male cardiovascular system: clinical and therapeutic implications in aging men.
Cho JJ, Cadet P, Salamon E, Mantione K, Stefano GB. Cho JJ, et al. Med Sci Monit. 2003 Mar;9(3):RA63-8. Med Sci Monit. 2003. PMID: 12640355 Review.
Cited by
- At What Point in the Menstrual Cycle Are the Pelvic Floor Muscles at Their Weakest?
Ojedo-Martín C, Rodríguez-López ES, Acevedo-Gómez MB, Úbeda-D'Ocasar E, de-Diego MV, Lara B. Ojedo-Martín C, et al. J Funct Morphol Kinesiol. 2024 Aug 8;9(3):135. doi: 10.3390/jfmk9030135. J Funct Morphol Kinesiol. 2024. PMID: 39189220 Free PMC article. - Mouse sarcopenia model reveals sex- and age-specific differences in phenotypic and molecular characteristics.
Kerr HL, Krumm K, Anderson B, Christiani A, Strait L, Li T, Irwin B, Jiang S, Rybachok A, Chen A, Dacek E, Caeiro L, Merrihew GE, MacDonald JW, Bammler TK, MacCoss MJ, Garcia JM. Kerr HL, et al. J Clin Invest. 2024 Jun 11;134(16):e172890. doi: 10.1172/JCI172890. J Clin Invest. 2024. PMID: 39145448 Free PMC article. - Influence of the Menstrual Cycle and Training on the Performance of a Perturbed Single-Leg Squatting Task in Female Collegiate Athletes.
Johnson KA, Shields RK. Johnson KA, et al. Orthop J Sports Med. 2024 Jun 2;12(6):23259671241251720. doi: 10.1177/23259671241251720. eCollection 2024 Jun. Orthop J Sports Med. 2024. PMID: 38831876 Free PMC article. - Muscle function, exercise capacity, physical activity level and cardiovascular disease risk factor knowledge in patients with prolactinoma.
Erkoç A, Eroğlu İ, Erbas T, Kutukcu EC. Erkoç A, et al. Endocrine. 2024 Sep;85(3):1337-1345. doi: 10.1007/s12020-024-03880-7. Epub 2024 May 27. Endocrine. 2024. PMID: 38801597 - Modified Squat Test for Predicting Knee Muscle Strength in Older Adults.
Tapanya W, Sangkarit N, Manoy P, Konsanit S. Tapanya W, et al. Ann Geriatr Med Res. 2024 Jun;28(2):209-218. doi: 10.4235/agmr.24.0005. Epub 2024 Apr 8. Ann Geriatr Med Res. 2024. PMID: 38584428 Free PMC article.
References
- Brzezinski A, Danenberg HD. Estrogen, progesterone, and cardiovascular health: when shall we complete the puzzle? Menopause. 2005;12:488–91. - PubMed
- Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. 2003;95:1717–27. - PubMed
- Felicio LS, Nelson JF, Finch CE. Longitudinal studies of estrous cyclicity in aging C57BL/6J mice: II. Cessation of cyclicity and the duration of persistent vaginal cornification. Biol Reprod. 1984;31:446–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K02 AG036827/AG/NIA NIH HHS/United States
- R01 AG031743-02/AG/NIA NIH HHS/United States
- AG031743/AG/NIA NIH HHS/United States
- K02 AG036827-01A1/AG/NIA NIH HHS/United States
- R01 AG031743/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical