An imbalance in mucosal cytokine profile causes transient intestinal inflammation following an animal's first exposure to faecal bacteria and antigens - PubMed (original) (raw)
An imbalance in mucosal cytokine profile causes transient intestinal inflammation following an animal's first exposure to faecal bacteria and antigens
B C Sydora et al. Clin Exp Immunol. 2010.
Abstract
Intestinal microflora play a critical role in the initiation and perpetuation of chronic inflammatory bowel diseases. In genetically susceptible hosts, bacterial colonization results in rapid-onset chronic intestinal inflammation. Nevertheless, the intestinal and systemic immune response to faecal bacteria and antigen exposure into a sterile intestinal lumen of a post-weaned animal with a mature immune system are not understood clearly. This study examined the effects of faecal bacteria and antigen exposure on the intestinal mucosal and systemic immune system in healthy axenic mice. Axenic wild-type mice were inoculated orally with a crude faecal slurry solution derived from conventionally raised mice and were analysed prior to and then at days 3, 7, 14 and 28 post-treatment. Ingestion of faecal slurry resulted in a transient, early onset of proinflammatory interferon (IFN)-gamma, tumour necrosis factor (TNF)-alpha and interleukin (IL)-17 response that was maximal at day 3. In contrast, the transient release of the anti-inflammatory cytokines IL-10 and IL-4 occurred later and was maximal at day 7. Both responses subsided by day 14. This early cytokine imbalance was associated with a brief rise in colonic and caecal histopathological injury score at day 7. The bacterial antigen-specific systemic response was found to follow the intestinal immune response with a maximal release of both pro- and anti-inflammatory cytokines at day 7. Thus, first exposure of healthy axenic wild-type mice to normal faecal flora and antigens results in an early proinflammatory cytokine response and transient colonic inflammation that then resolves in conjunction with a subsequent anti-inflammatory cytokine profile.
Figures
Fig. 1
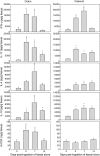
Kinetics of cytokine release from colonic and caecal mucosal explant cultures derived from axenic 129/SvEv wild-type mice following ingestion of faecal slurry. Spontaneous release of interferon (IFN)-γ, interleukin (IL)-17, IL-4, IL-10 and granulocyte-colony stimulating factor (G-CSF) was assessed in supernatants after 24 h using standard enzyme-linked immunosorbent assay (ELISA) techniques. Production of proinflammatory cytokines (IFN-γ, IL-17) as well as production of G-CSF preceded the secretion of anti-inflammatory cytokines (IL-14, IL-10). Bars represent mean values ± standard error of the mean for four to six mice per group; *P > 0·05.
Fig. 2
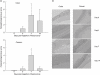
Histological injury assessment in Swiss Webster mice prior to and on days 3, 7 and 14 post-faecal slurry ingestion. Injury score (a) and histological inflammation (b) in both colon and caecum is maximal at day 7 and declines thereafter. Values represent mean values ± standard error of the mean for five mice per group. Fig. 2b shows haematoxylin and eosin staining of a histology preparation from a representative mouse for each time-point.
Fig. 3
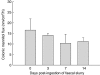
Epithelial barrier integrity as measured by colonic transmural mannitol flux in Ussing chambers pre- and post-faecal slurry ingestion. Bars represent mean values ± standard error of the mean for three Swiss Webster mice per group. All mice were assessed in duplicate.
Fig. 4
Systemic immune response prior to and on days 3, 7 and 14 following faecal slurry ingestion as measured by cytokine release in 24-h antigen-stimulated cultures of spleen cells from 129/SvEv mice. At day 7 a temporary maximal increase in cytokine production [interferon-γ, interleukin (IL)-17 and IL-10, but not IL-4] was observed in response to (a) sterile lysates prepared from caecal matter of specific pathogen-free mice containing several endogenous intestinal bacteria to (b) sterile bacterial antigens prepared from pure cultures of Bacteroides vulgatus, Enterococcus cloacae and Lactobacillus reuteri and to (c) cross-linked anti-CD3 monoclonal antibodies. The results from stimulation with the three bacterial antigens are combined in one bar. Values represent mean values ± standard error of the mean for five to 10 mice per group; *P > 0·05.
Fig. 4
Systemic immune response prior to and on days 3, 7 and 14 following faecal slurry ingestion as measured by cytokine release in 24-h antigen-stimulated cultures of spleen cells from 129/SvEv mice. At day 7 a temporary maximal increase in cytokine production [interferon-γ, interleukin (IL)-17 and IL-10, but not IL-4] was observed in response to (a) sterile lysates prepared from caecal matter of specific pathogen-free mice containing several endogenous intestinal bacteria to (b) sterile bacterial antigens prepared from pure cultures of Bacteroides vulgatus, Enterococcus cloacae and Lactobacillus reuteri and to (c) cross-linked anti-CD3 monoclonal antibodies. The results from stimulation with the three bacterial antigens are combined in one bar. Values represent mean values ± standard error of the mean for five to 10 mice per group; *P > 0·05.
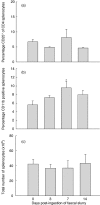
Fig. 5
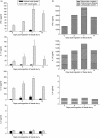
Flow cytometric assessment and enumeration of spleen cells pre- and post-faecal slurry ingestion. No significant increase in the population of CD25-positive cells was noted (a). At day 7 CD11b-positive leucocytes increased significantly in the spleen cell population (b). The total number of spleen cells remained similar at various time-points post-feeding (c). Values represent mean values ± standard error of the mean (s.e.m.) for three to four mice per group (a) and mean values ± s.e.m. for five to 10 mice per group (b,c); *P > 0·05. Results for CD25 staining were derived from one experiment with Swiss Webster mice. Results for CD11b staining as well as spleen cell enumeration data were derived from experiments with 129/SvEv mice; however, data from Swiss Webster mice generated similar results.
Similar articles
- Neonatal exposure to fecal antigens reduces intestinal inflammation.
Sydora BC, McFarlane SM, Doyle JS, Fedorak RN. Sydora BC, et al. Inflamm Bowel Dis. 2011 Apr;17(4):899-906. doi: 10.1002/ibd.21453. Epub 2010 Sep 7. Inflamm Bowel Dis. 2011. PMID: 20824814 - Microflora reactive IL-10 producing regulatory T cells are present in the colon of IL-2 deficient mice but lack efficacious inhibition of IFN-gamma and TNF-alpha production.
Waidmann M, Allemand Y, Lehmann J, di Genaro S, Bücheler N, Hamann A, Autenrieth IB. Waidmann M, et al. Gut. 2002 Feb;50(2):170-9. doi: 10.1136/gut.50.2.170. Gut. 2002. PMID: 11788555 Free PMC article. - Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora.
Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Madsen KL, et al. Inflamm Bowel Dis. 1999 Nov;5(4):262-70. doi: 10.1097/00054725-199911000-00004. Inflamm Bowel Dis. 1999. PMID: 10579119 - Alterations of the mucosal immune system in inflammatory bowel disease.
MacDermott RP. MacDermott RP. J Gastroenterol. 1996 Dec;31(6):907-16. doi: 10.1007/BF02358624. J Gastroenterol. 1996. PMID: 9027661 Review.
Cited by
- Hepatocyte growth factor ameliorates dextran sodium sulfate‑induced colitis in a mouse model by altering the phenotype of intestinal macrophages.
Fujino Y, Kanmura S, Morinaga Y, Kojima I, Maeda N, Tanaka A, Maeda H, Kumagai K, Sasaki F, Tanoue S, Ido A. Fujino Y, et al. Mol Med Rep. 2023 Mar;27(3):70. doi: 10.3892/mmr.2023.12957. Epub 2023 Feb 17. Mol Med Rep. 2023. PMID: 36799161 Free PMC article. - Oral administration of Lactobacillus plantarum 06CC2 prevents experimental colitis in mice via an anti‑inflammatory response.
Tanaka A, Kanmura S, Morinaga Y, Kawabata K, Arima S, Sasaki F, Nasu Y, Tanoue S, Hashimoto S, Takeshita M, Takeda S, Ido A. Tanaka A, et al. Mol Med Rep. 2020 Mar;21(3):1181-1191. doi: 10.3892/mmr.2020.10925. Epub 2020 Jan 9. Mol Med Rep. 2020. PMID: 31922249 Free PMC article. - Extracellular Vesicles Derived from Bone Marrow Mesenchymal Stem Cells Protect against Experimental Colitis via Attenuating Colon Inflammation, Oxidative Stress and Apoptosis.
Yang J, Liu XX, Fan H, Tang Q, Shou ZX, Zuo DM, Zou Z, Xu M, Chen QY, Peng Y, Deng SJ, Liu YJ. Yang J, et al. PLoS One. 2015 Oct 15;10(10):e0140551. doi: 10.1371/journal.pone.0140551. eCollection 2015. PLoS One. 2015. PMID: 26469068 Free PMC article. - Microbiota maintain colonic homeostasis by activating TLR2/MyD88/PI3K signaling in IL-10-producing regulatory B cells.
Mishima Y, Oka A, Liu B, Herzog JW, Eun CS, Fan TJ, Bulik-Sullivan E, Carroll IM, Hansen JJ, Chen L, Wilson JE, Fisher NC, Ting JP, Nochi T, Wahl A, Garcia JV, Karp CL, Sartor RB. Mishima Y, et al. J Clin Invest. 2019 Jun 18;129(9):3702-3716. doi: 10.1172/JCI93820. J Clin Invest. 2019. PMID: 31211700 Free PMC article. - Effect of solar particle event radiation on gastrointestinal tract bacterial translocation and immune activation.
Ni H, Balint K, Zhou Y, Gridley DS, Maks C, Kennedy AR, Weissman D. Ni H, et al. Radiat Res. 2011 Apr;175(4):485-92. doi: 10.1667/RR2373.1. Epub 2011 Feb 4. Radiat Res. 2011. PMID: 21294608 Free PMC article.
References
- Hughes KL. Recent knowledge of the strict anaerobes of the gut. Aust Vet J. 1972;48:508–14. - PubMed
- Sartor RB. Targeting enteric bacteria in treatment of inflammatory bowel diseases: why, how, and when. Curr Opin Gastroenterol. 2003;19:358–65. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical