Purified TPC isoforms form NAADP receptors with distinct roles for Ca(2+) signaling and endolysosomal trafficking - PubMed (original) (raw)
. 2010 Apr 27;20(8):703-9.
doi: 10.1016/j.cub.2010.02.049. Epub 2010 Mar 25.
Katja Rietdorf, Abdelilah Arredouani, Lianne C Davis, Emyr Lloyd-Evans, Heidi Koegel, Timothy M Funnell, Anthony J Morgan, John A Ward, Keiko Watanabe, Xiaotong Cheng, Grant C Churchill, Michael X Zhu, Frances M Platt, Gary M Wessel, John Parrington, Antony Galione
Affiliations
- PMID: 20346675
- PMCID: PMC2861162
- DOI: 10.1016/j.cub.2010.02.049
Purified TPC isoforms form NAADP receptors with distinct roles for Ca(2+) signaling and endolysosomal trafficking
Margarida Ruas et al. Curr Biol. 2010.
Abstract
Intracellular Ca(2+) signals constitute key elements in signal transduction. Of the three major Ca(2+) mobilizing messengers described, the most potent, nicotinic acid adenine dinucleotide phosphate (NAADP) is the least well understood in terms of its molecular targets [1]. Recently, we showed that heterologous expression of two-pore channel (TPC) proteins enhances NAADP-induced Ca(2+) release, whereas the NAADP response was abolished in pancreatic beta cells from Tpcn2 gene knockout mice [2]. However, whether TPCs constitute native NAADP receptors is unclear. Here we show that immunopurified endogenous TPC complexes possess the hallmark properties ascribed to NAADP receptors, including nanomolar ligand affinity [3-5]. Our study also reveals important functional differences between the three TPC isoforms. Thus, TPC1 and TPC2 both mediate NAADP-induced Ca(2+) release, but the subsequent amplification of this trigger Ca(2+) by IP(3)Rs is more tightly coupled for TPC2. In contrast, TPC3 expression suppressed NAADP-induced Ca(2+) release. Finally, increased TPC expression has dramatic and contrasting effects on endolysosomal structures and dynamics, implicating a role for NAADP in the regulation of vesicular trafficking. We propose that NAADP regulates endolysosomal Ca(2+) storage and release via TPCs and coordinates endoplasmic reticulum Ca(2+) release in a role that impacts on Ca(2+) signaling in health and disease [6].
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
Figure 1
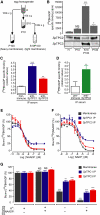
Endogenous _Sp_TPC Protein Complexes Bind to [32P]NAADP with Hallmark Properties of the NAADP Receptor (A) Schematic representation of protocol used for membrane preparations from Strongylocentrotus purpuratus egg homogenates via differential centrifugation. The following abbreviations are used: H, homogenate; S, supernatant; P, pellet. (B) Levels of specific [32P]NAADP binding in membrane preparations (n = 3). Panels below graph correspond to immunoblots probed with affinity-purified _Sp_TPC isoform-specific antibodies showing high molecular weight forms of _Sp_TPCs. (C) Specific [32P]NAADP binding for _Sp_TPC1 and _Sp_TPC3 immunoprecipitates (IPs) from S10P100 fractions with anti-_Sp_TPC1 and anti-_Sp_TPC3 sera. Values (n = 6) determined by Cerenkov counting were normalized against control IP with nonimmune serum. (D) Specific [32P]NAADP binding for _Sp_TPC2 IP from P100 fractions with anti-_Sp_TPC2 serum. Values (n = 11) obtained by phosphorimaging were normalized against control IP with preimmune serum. (E) Inhibition of [32P]NAADP binding to S10P100 membranes or _Sp_TPC1 or _Sp_TPC3 IPs by increasing concentrations of NAADP (n = 3). Values were normalized to amount of bound [32P]NAADP in absence of competitor. (F) Same as in (E), but with NADP as competitor (n = 3). (G) Bound [32P]NAADP to S10P100 membranes or _Sp_TPC1 or _Sp_TPC3 IPs after washes with buffer with K+ (GluIM) or without K+ (HEPES), in the absence or presence of 10 μM NAADP. Values (n = 3) of remaining radioactivity were normalized to values obtained for GluIM washes in the absence of NAADP. Data are represented as mean ± standard error of the mean (SEM); p > 0.05 (not significant, NS), ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figure S1.
Figure 2
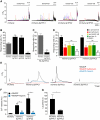
NAADP-Mediated Ca2+ Release in Mammalian Cells Expressing _Sp_TPCs (A) Ca2+ traces of mCherry._Sp_TPC-expressing cells stimulated by 1 μM NAADP-AM. ATP (100 μM) was added at the end of each experiment to assess cell viability. The number of cells not responding to ATP was negligible. Traces of cells responding to NAADP-AM (F/F0 > 1.5) are shown in color. (B) Summary of results from experiments performed over 4 days with 12–22 coverslips per cell line, as outlined in (A). Only cells responding to ATP were included in the data analysis. (C) Effect of mCherry._Sp_TPC3 transient expression on cells stably expressing HA._Sp_TPC2. Transfection of mCherry alone is included as control. Summary of results is from experiments performed over 2 days with 4–6 coverslips per transfection. There were no significant differences between untransfected cells and cells transfected with mCherry alone (data not shown). (D) Effect of pretreatment with NAADP-AM (1 nM, 30 min), bafilomycin A1 (3 μM, 60 min), or trans Ned-19 (10 μM, 15 min) upon 1 μM NAADP-AM-induced Ca2+ release in cells expressing mCherry._Sp_TPC1 or mCherry._Sp_TPC2. Spontaneous activity was determined by addition of dimethyl sulfoxide (DMSO). Results from 2–3 days of experiments with 6–17 coverslips for each treatment and cell line are shown. (E) Representative Ca2+ traces of cells dialyzed with NAADP (100 nM) via patch pipettes in whole-cell configuration, in the absence or presence of bafilomycin A1 (1 μM) or heparin (200 μg/ml). Arrows indicate break-in. In total, 5–7 cells per condition and cell line were analyzed, and all of the cells apart from _Sp_TPC3 were responsive to NAADP dialysis, with _Sp_TPC1 and _Sp_TPC2 giving a biphasic response. (F) Summary of results from (E), quantifying total Ca2+ release as area under curve. (G) Summary of results from (E), quantifying the time required to trigger the secondary Ca2+ response after break-in. Data are represented as mean ± SEM; p > 0.05 (NS), ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figure S2.
Figure 3
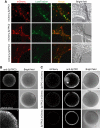
Intracellular Localization of _Sp_TPCs (A) Colocalization of mCherry._Sp_TPC with acidic organelles (LysoTracker Green) in live HEK293 cells expressing mCherry._Sp_TPCs. Scale bar corresponds to 10 μm. (B) Anti-_Sp_TPC3 immunofluorescence of S. purpuratus eggs with or without peptide block for assessment of immunostaining specificity. Scale bar corresponds to 10 μm, or 2 μm for higher magnification panel. (C) Localization of _Sp_TPCs.mCherry expressed in oocytes of starfish Asterina miniata and detected by mCherry fluorescence or by immunofluorescence with corresponding anti-_Sp_TPC antibodies. Scale bar corresponds to 20 μm. Noninjected controls were not labeled with anti-_Sp_TPC antibodies (data not shown). In oocytes expressing _Sp_TPC3.mCherry, the germinal vesicle is out of the plane of focus. mCherry._Sp_TPCs showed similar cortical localization (data not shown). Oocytes expressing mCherry alone showed a signal across the entire cell (data not shown). Of note is the presence of an _Sp_TPC1 signal in the nucleus. Indeed, _Sp_TPC1 has a putative nuclear localization signal (amino acids 327–334). Its physiological significance may represent an important avenue for further analysis of TPC function, especially in light of reports describing NAADP signaling in nucleus [33, 34]. See also Figure S3.
Figure 4
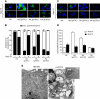
Two-Pore Channel Overexpression Causes Changes in Endolysosomal Trafficking and Morphology (A) Lipid endocytosis and recycling in HA._Sp_TPC-overexpressing HEK293 cells assessed by Alexa Fluor 568-cholera toxin B subunit (CtxB; green). Nuclei are labeled with Hoechst 33342 (Hoechst, blue). Scale bar corresponds to 5 μm. Endolysosomal localization was confirmed by colocalization with endocytosed high molecular weight rhodamine dextran (data not shown); Golgi localization by a single perinuclear distribution is not overlapping with the dextran. (B) Summary of results from experiments in (A) corresponding to three separate experiments with a minimum of 50 cells analyzed per experiment. (C) Pattern of lysosomal staining by LysoTracker Green (LysoTracker, green) in HA._Sp_TPC-overexpressing HEK293 cells, in the absence or presence of NAADP receptor antagonist Ned-19 (10 μM, 12 hr). Nuclei were labeled with Hoechst 33342 (Hoechst, blue). Scale bars correspond to 5 μm. (D) Summary of results from experiments in (C) corresponding to three separate experiments, with a minimum of 50 cells analyzed per experiment. To allow meaningful comparison between images, we standardized LysoTracker loading conditions and imaging protocols for all cell types. pH differences between cell types were negligible, as assessed by fluorescein-dextran fluorescence (data not shown). (E) Lysosomal storage disease phenotype in HA._Hs_TPC2-overexpressing HEK293 cells visualized by electron microscopy. Inset is a magnification of one region of a HA._Hs_TPC2-overexpressing cell. Scale bars correspond to 200 nm. The following abbreviations are used: Nuc, nucleus; MLB, multiple lamellar inclusion body; L, lysosome. Data are represented as mean ± SEM. See also Figure S4.
Similar articles
- Calcium signaling via two-pore channels: local or global, that is the question.
Zhu MX, Ma J, Parrington J, Calcraft PJ, Galione A, Evans AM. Zhu MX, et al. Am J Physiol Cell Physiol. 2010 Mar;298(3):C430-41. doi: 10.1152/ajpcell.00475.2009. Epub 2009 Dec 16. Am J Physiol Cell Physiol. 2010. PMID: 20018950 Free PMC article. Review. - NAADP as an intracellular messenger regulating lysosomal calcium-release channels.
Galione A, Morgan AJ, Arredouani A, Davis LC, Rietdorf K, Ruas M, Parrington J. Galione A, et al. Biochem Soc Trans. 2010 Dec;38(6):1424-31. doi: 10.1042/BST0381424. Biochem Soc Trans. 2010. PMID: 21118101 - Both RyRs and TPCs are required for NAADP-induced intracellular Ca²⁺ release.
Gerasimenko JV, Charlesworth RM, Sherwood MW, Ferdek PE, Mikoshiba K, Parrington J, Petersen OH, Gerasimenko OV. Gerasimenko JV, et al. Cell Calcium. 2015 Sep;58(3):237-45. doi: 10.1016/j.ceca.2015.05.005. Epub 2015 Jun 10. Cell Calcium. 2015. PMID: 26100948 Free PMC article. - NAADP mobilizes calcium from acidic organelles through two-pore channels.
Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt CN, Parrington J, Ma J, Evans AM, Galione A, Zhu MX. Calcraft PJ, et al. Nature. 2009 May 28;459(7246):596-600. doi: 10.1038/nature08030. Epub 2009 Apr 22. Nature. 2009. PMID: 19387438 Free PMC article. - NAADP receptors.
Galione A. Galione A. Cold Spring Harb Perspect Biol. 2011 Jan 1;3(1):a004036. doi: 10.1101/cshperspect.a004036. Cold Spring Harb Perspect Biol. 2011. PMID: 21047915 Free PMC article. Review.
Cited by
- Intracellular two-phase Ca2+ release and apoptosis controlled by TRP-ML1 channel activity in coronary arterial myocytes.
Xu M, Li X, Walsh SW, Zhang Y, Abais JM, Boini KM, Li PL. Xu M, et al. Am J Physiol Cell Physiol. 2013 Mar 1;304(5):C458-66. doi: 10.1152/ajpcell.00342.2012. Epub 2013 Jan 2. Am J Physiol Cell Physiol. 2013. PMID: 23283937 Free PMC article. - TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes.
Wang X, Zhang X, Dong XP, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, Zhu MX, Clapham DE, Ren D, Xu H. Wang X, et al. Cell. 2012 Oct 12;151(2):372-83. doi: 10.1016/j.cell.2012.08.036. Cell. 2012. PMID: 23063126 Free PMC article. - Leucine-rich repeat kinase 2 regulates autophagy through a calcium-dependent pathway involving NAADP.
Gómez-Suaga P, Luzón-Toro B, Churamani D, Zhang L, Bloor-Young D, Patel S, Woodman PG, Churchill GC, Hilfiker S. Gómez-Suaga P, et al. Hum Mol Genet. 2012 Feb 1;21(3):511-25. doi: 10.1093/hmg/ddr481. Epub 2011 Oct 19. Hum Mol Genet. 2012. PMID: 22012985 Free PMC article. - Sex-dependent calcium hyperactivity due to lysosomal-related dysfunction in astrocytes from APOE4 versus APOE3 gene targeted replacement mice.
Larramona-Arcas R, González-Arias C, Perea G, Gutiérrez A, Vitorica J, García-Barrera T, Gómez-Ariza JL, Pascua-Maestro R, Ganfornina MD, Kara E, Hudry E, Martinez-Vicente M, Vila M, Galea E, Masgrau R. Larramona-Arcas R, et al. Mol Neurodegener. 2020 Jun 9;15(1):35. doi: 10.1186/s13024-020-00382-8. Mol Neurodegener. 2020. PMID: 32517777 Free PMC article. - Direct Visualization of Ebola Virus Fusion Triggering in the Endocytic Pathway.
Spence JS, Krause TB, Mittler E, Jangra RK, Chandran K. Spence JS, et al. mBio. 2016 Feb 9;7(1):e01857-15. doi: 10.1128/mBio.01857-15. mBio. 2016. PMID: 26861015 Free PMC article.
References
- Galione A., Petersen O.H. The NAADP receptor: New receptors or new regulation? Mol. Interv. 2005;5:73–79. - PubMed
- Aarhus R., Dickey D.M., Graeff R.M., Gee K.R., Walseth T.F., Lee H.C. Activation and inactivation of Ca2+ release by NAADP+ J. Biol. Chem. 1996;271:8513–8516. - PubMed
- Billington R.A., Genazzani A.A. Characterization of NAADP+ binding in sea urchin eggs. Biochem. Biophys. Res. Commun. 2000;276:112–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous