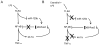Estradiol suppresses NF-kappa B activation through coordinated regulation of let-7a and miR-125b in primary human macrophages - PubMed (original) (raw)
Estradiol suppresses NF-kappa B activation through coordinated regulation of let-7a and miR-125b in primary human macrophages
Amy J Murphy et al. J Immunol. 2010.
Abstract
Previous findings suggest that 17beta-estradiol (estradiol) has a suppressive effect on TNF-alpha, but the mechanism by which estradiol regulates TNF-alpha expression in primary human macrophages is unknown. In this article, we demonstrate that pretreatment of human macrophages with estradiol attenuates LPS-induced TNF-alpha expression through the suppression of NF-kappaB activation. Furthermore, we show that activation of macrophages with LPS decreases the expression of kappaB-Ras2, an inhibitor of NF-kappaB signaling. Estradiol pretreatment abrogates this decrease, leading to the enhanced expression of kappaB-Ras2 with LPS stimulation. Additionally, we identified two microRNAs, let-7a and miR-125b, which target the kappaB-Ras2 3' untranslated region (UTR). LPS induces let-7a and inhibits miR-125b expression in human macrophages, and pretreatment with estradiol abrogates these effects. 3'UTR reporter assays demonstrate that let-7a destabilizes the kappaB-Ras2 3'UTR, whereas miR-125b enhances its stability, resulting in decreased kappaB-Ras2 in response to LPS. Our data suggest that pretreatment with estradiol reverses this effect. We propose a novel mechanism for estradiol inhibition of LPS-induced NF-kappaB signaling in which kappaB-Ras2 expression is induced by estradiol via regulation of let-7a and miR-125b. These findings are significant in that they are the first to demonstrate that estradiol represses NF-kappaB activation through the induction of kappaB-Ras2, a key inhibitor of NF-kappaB signaling.
Figures
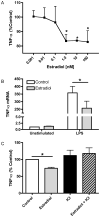
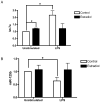
Figure 1. Estradiol attenuates LPS-induced TNF-α
(A) ELISA analysis of TNF-α levels in culture supernatants from macrophages treated with estradiol or ethanol control for 24 hours prior to stimulation with LPS (10 ng/ml) for 12 hours. Data are presented as percent of TNF-α produced by LPS-stimulated cells not receiving estradiol treatment. (B) Total RNA was extracted from macrophages treated with or without estradiol (100 nM) followed by stimulation with LPS for 5 hours. TNF-α mRNA was measured by TaqMan real-time PCR. (C) Macrophages were treated with the estrogen receptor antagonist ICI 182,780 (1 μM) for 1 hour prior to estradiol treatment. Hormone and LPS treatments were the same as in A. TNF-α levels were measured by ELISA and are presented as percent of control treated cells stimulated with LPS. *p<0.05 vs. control cells stimulated with LPS.
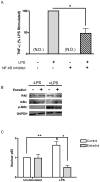
Figure 2. Estradiol inhibits NF-κB signaling
(A) Cells were treated with NF-κB activation inhibitor (10 μM) for 1 hour followed by LPS stimulation for 5 hours. TNF-α levels in culture supernatants were measured by ELISA. N.D., not detected. (B) Whole cell lysates were prepared from macrophages pre-treated with estradiol and then stimulated with LPS for 1 hour. IKKβ, IκBα and phospho-IκBα levels were measured by immunoblot and GAPDH served as a loading control. (C) Cells were treated as in B, and nuclear lysates were prepared and analyzed for p65 by ELISA. Data are normalized to control treated, unstimulated lev28ls. *p<0.01 vs. LPS stimulation alone. **p<0.05 vs. control treated and unstimulated.
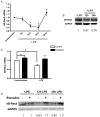
Figure 3. κB-Ras2 is up-regulated by estradiol
(A) Total RNA was extracted from macrophages treated with LPS at indicated times. κB-Ras2 mRNA was measured by TaqMan real-time PCR. Values are relative to unstimulated controls. (B) Immunoblot analysis of κB-Ras2 protein levels in response to LPS. (C–D) mRNA and protein levels, respectively, of κB-Ras2. Macrophages were treated with estradiol prior to LPS stimulation for 8 hours (C) or 12–24 hours (D). Data shown are representative of 4 experiments. *p< 0.05 vs. unstimulated control
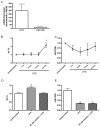
Figure 4. Let-7a and miR-125b are regulated by LPS
(A) Total RNA was extracted from macrophages using Qiagen miRNeasy kits. Let-7a and miR-125b expression were measured using Taqman miRNA assay system. Data are normalized to U6 expression levels. (B) let-7a and (C) miR-125b expression levels in response to LPS stimulation. Data were normalized to U6 and are presented as relative to unstimulated levels. (D–E) Macrophages from a representative donor were treated with NF-κB activation inhibitor (10 μM) for 1 hour prior to LPS stimulation for (D) 12 hours or (E) 3 hours. *p<0.05 vs. unstimulated controls.
Figure 5. Estradiol inhibits LPS effect on let-7a and miR-125b
(A) let-7a levels in macrophages pre-treated with estradiol and stimulated with LPS for 12 hours. (B) miR-125b expression in cells treated with estradiol and stimulated with LPS for 3 hours. *p<0.05 vs. unstimulated controls.
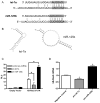
Figure 6. let-7a and miR-125b directly target kB-Ras2 3′UTR
(A) Alignment of let-7a and miR-125b with κB-Ras2 3′UTR. Solid lines indicate Watson-Crick base pairs. Dotted line indicates GU wobble pairs. The gray background denotes the seed binding region. (B) Secondary structure as predicted by RNAHybrid with κB-Ras2 3′UTR shown in black and miRNA in gray. (C) RAW 264.7 cells were transfected with luciferase expression vector containing the κB-Ras2 3′UTR or the control vector and synthetic pre-miR-125b, pre-let-7a or a negative control pre-miRNA. The cells were also transfected with a vector containing Renilla luciferase to serve as a transfection efficiency control. After 48 hours, cells were lysed and analyzed for luciferase expression using a dual-luciferase assay system. (D) PMA-differentiated U937 cells were transfected with pre-miR-125b, pre-let-7a or pre-miRNA negative control and analyzed for κB-Ras2 expression after 48 hours. *p<0.05 vs. negative control miRNA.
Figure 7. Proposed mechanism of estradiol regulation of NF-κB signaling in primary human macrophages
(A) LPS binding to TLR4 induces expression of let-7a and decreases expression of miR-125b, leading to decreased expression of κB-Ras2 to enable complete activation of NF-κB signaling and TNF-α expression. (B) When macrophages are treated with estradiol prior to LPS stimulation, changes in expression of let-7a and miR-125b in response to 29LPS are abrogated. This results in up-regulation of κB-Ras2 and inhibition of NF-κB signaling, culminating in decreased expression of TNF-α.
Similar articles
- Induction of in vitro reprogramming by Toll-like receptor (TLR)2 and TLR4 agonists in murine macrophages: effects of TLR "homotolerance" versus "heterotolerance" on NF-kappa B signaling pathway components.
Dobrovolskaia MA, Medvedev AE, Thomas KE, Cuesta N, Toshchakov V, Ren T, Cody MJ, Michalek SM, Rice NR, Vogel SN. Dobrovolskaia MA, et al. J Immunol. 2003 Jan 1;170(1):508-19. doi: 10.4049/jimmunol.170.1.508. J Immunol. 2003. PMID: 12496438 - Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock.
Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, Croce CM. Tili E, et al. J Immunol. 2007 Oct 15;179(8):5082-9. doi: 10.4049/jimmunol.179.8.5082. J Immunol. 2007. PMID: 17911593 - Evidence for a dual mechanism for IL-10 suppression of TNF-alpha production that does not involve inhibition of p38 mitogen-activated protein kinase or NF-kappa B in primary human macrophages.
Denys A, Udalova IA, Smith C, Williams LM, Ciesielski CJ, Campbell J, Andrews C, Kwaitkowski D, Foxwell BM. Denys A, et al. J Immunol. 2002 May 15;168(10):4837-45. doi: 10.4049/jimmunol.168.10.4837. J Immunol. 2002. PMID: 11994432 - MicroRNA-26b Modulates the NF-κB Pathway in Alveolar Macrophages by Regulating PTEN.
Zhang L, Huang C, Guo Y, Gou X, Hinsdale M, Lloyd P, Liu L. Zhang L, et al. J Immunol. 2015 Dec 1;195(11):5404-14. doi: 10.4049/jimmunol.1402933. Epub 2015 Oct 26. J Immunol. 2015. PMID: 26503952 Free PMC article.
Cited by
- Protein Kinases Mediate Anti-Inflammatory Effects of Cannabidiol and Estradiol Against High Glucose in Cardiac Sodium Channels.
Fouda MA, Ruben PC. Fouda MA, et al. Front Pharmacol. 2021 Apr 28;12:668657. doi: 10.3389/fphar.2021.668657. eCollection 2021. Front Pharmacol. 2021. PMID: 33995099 Free PMC article. - Interplay between estrogen and Stat3/NF-κB-driven immunomodulation in lung cancer.
Deng S, Ramos-Castaneda M, Velasco WV, Clowers MJ, Gutierrez BA, Noble O, Dong Y, Zarghooni M, Alvarado L, Caetano MS, Yang S, Ostrin EJ, Behrens C, Wistuba II, Stabile LP, Kadara H, Watowich SS, Moghaddam SJ. Deng S, et al. Carcinogenesis. 2020 Nov 13;41(11):1529-1542. doi: 10.1093/carcin/bgaa064. Carcinogenesis. 2020. PMID: 32603404 Free PMC article. - LncRNA AK089514/miR-125b-5p/TRAF6 axis mediates macrophage polarization in allergic asthma.
Zhu X, He L, Li X, Pei W, Yang H, Zhong M, Zhang M, Lv K, Zhang Y. Zhu X, et al. BMC Pulm Med. 2023 Jan 30;23(1):45. doi: 10.1186/s12890-023-02339-1. BMC Pulm Med. 2023. PMID: 36717790 Free PMC article. - Regulation of breast cancer and bone metastasis by microRNAs.
Vimalraj S, Miranda PJ, Ramyakrishna B, Selvamurugan N. Vimalraj S, et al. Dis Markers. 2013;35(5):369-87. doi: 10.1155/2013/451248. Epub 2013 Sep 26. Dis Markers. 2013. PMID: 24191129 Free PMC article. Review. - Bax Targeted by miR-29a Regulates Chondrocyte Apoptosis in Osteoarthritis.
Miao G, Zang X, Hou H, Sun H, Wang L, Zhang T, Tan Y, Liu W, Ye P, Gao L, Zha Z. Miao G, et al. Biomed Res Int. 2019 Mar 12;2019:1434538. doi: 10.1155/2019/1434538. eCollection 2019. Biomed Res Int. 2019. PMID: 30993110 Free PMC article.
References
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. - PubMed
- Billiau A, Vandekerckhove F. Cytokines and their interactions with other inflammatory mediators in the pathogenesis of sepsis and septic shock. Eur J Clin Invest. 1991;21:559–573. - PubMed
- Zandman-Goddard G, Peeva E, Shoenfeld Y. Gender and autoimmunity. Autoimmunity Reviews. 2007;6:366–372. - PubMed
- Vural P, Akgul C, Canbaz M. Effects of hormone replacement therapy on plasma pro-inflammatory and anti-inflammatory cytokines and some bone turnover markers in postmenopausal women. Pharmacological Research. 2006;54:298–302. - PubMed
- Schröder J, Kahlke V, Staubach KH, Zabel P, Stüber F. Gender differences in human sepsis. Archives of surgery (Chicago, Ill: 1960) 1998;133:1200–1205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR016437/RR/NCRR NIH HHS/United States
- P20 RR 016437/RR/NCRR NIH HHS/United States
- R01 AI051547/AI/NIAID NIH HHS/United States
- P30 CA023108/CA/NCI NIH HHS/United States
- T32 AR007576/AR/NIAMS NIH HHS/United States
- T32AR007576/AR/NIAMS NIH HHS/United States
- R01AI051547/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources