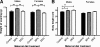Perinatal exposure to bisphenol-a and the development of metabolic syndrome in CD-1 mice - PubMed (original) (raw)
Perinatal exposure to bisphenol-a and the development of metabolic syndrome in CD-1 mice
Karen K Ryan et al. Endocrinology. 2010 Jun.
Abstract
Bisphenol-A (BPA) is an endocrine-disrupting chemical used in the production of plastic food and beverage containers, leading to ubiquitous low-dose human exposure. It has been suggested that exposure to even low doses of BPA during development may be associated with increased susceptibility to obesity and diabetes later in life. Despite growing public concern, the existing empirical data are equivocal, prompting The Endocrine Society, the National Institute of Environmental Health Sciences, and others to call for further research. In this study, we tested the hypothesis that perinatal exposure to an ecologically relevant dose of BPA (1 part per billion via the diet) results in increased susceptibility to high-fat diet-induced obesity and glucose intolerance in adult CD-1 mice. The data did not support this hypothesis. In agreement with previous reports, we find that weanling mice exposed to BPA during gestation and lactation are heavier compared with control mice. We also find that BPA mice are longer than controls at 4 wk of age, but these differences are no longer apparent when the mice reach adulthood, even when tested on a high-fat diet. We conclude that this larger size-for-age represents a faster rate of growth early in development rather than an obese, diabetic phenotype in adulthood.
Figures
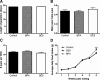
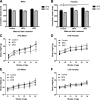
Figure 1
Effects of perinatal exposure to BPA and DES on traits of dams and their litters. Length of gestation in days (e0–p0) (A), sex ratio (B), and litter size (C) of dams eating either control diet (n = 31) or diet containing 4-ppb BPA (n = 34) or 1-ppb DES (n = 32). There were no significant differences among the groups for these endpoints (ANOVA, P > 0.05). D, Average daily food intake of dams (and their pups) exposed to BPA is higher at wk 6 compared with mice eating the control or DES diets [repeated-measures (RM) ANCOVA followed by Tukey post hoc tests: P (litter size) <0.000001, P (time × maternal treatment) <0.000001; within wk 6, P (BPA vs. control) <0.001, P (BPA vs. DES) <0.001]. ***, P < 0.001.
Figure 2
Body size of weanlings. A, Male and female BPA pups were heavier at 3 wk of age compared with control and DES pups [ANCOVA followed by Tukey post hoc tests: for males, P (maternal treatment) <0.000001, P (BPA vs. control) <0.01, P (BPA vs. DES) <0.01, P (litter size) <0.000001; for females, P (maternal treatment) <0.000001, P (BPA vs. control) <0.01, P (BPA vs. DES) <0.001, P (litter size) <0.000001]. B, Body length was measured in a subset of animals (n = 10 sex/maternal treatment) at 4 wk of age. Among males, control mice were significantly shorter than both BPA and DES mice. Among females, the trend was similar, but the differences were not significant when litter size was included in the model [ANCOVA followed by Tukey post hoc tests: for males, P (maternal treatment) <0.01, P (control vs. BPA) <0.05, P (control vs. DES) <0.01, P (litter size) <0.059; for females, P (maternal treatment) <0.098, P (litter size) <0.05]. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
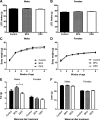
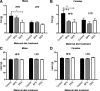
Figure 3
Growth on LFD from 4–9 wk of age. Cumulative LFD intake by male (A) and female (B) mice did not differ according to maternal treatment [ANCOVA: P (maternal treatment) >0.05, P (litter size) >0.05]. C, Among males, body weight differences among the maternal treatment groups were lost after weaning [repeated-measures (RM) ANCOVA: P (maternal treatment) >0.05, P (litter size) <0.01, _P_ (litter size × time) <0.01]. D, Among females, body weight differences among the maternal treatment groups were lost after weaning [RM ANCOVA: _P_ (maternal treatment) >0.05, P (litter size) <0.01, _P_ (litter size × time) <0.001, _P_ (maternal treatment × time) <0.000001]. E, At 7 wk of age, BPA males had more body fat than DES males [ANCOVA followed by Tukey _post hoc_ tests: _P_ (maternal treatment) <0.05, _P_ (BPA _vs_. DES) <0.05, _P_ (litter size) >0.05]. At 7 wk of age, there were no body fat differences among the females [ANCOVA: P (maternal treatment) >0.05, P (litter size) >0.05]. F, There were no differences in lean mass among the groups for either sex [ANCOVA: P (maternal treatment) >0.05, P (litter size) >0.05]. *, P < 0.05.
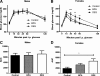
Figure 4
Blood glucose in 8-wk-old male and female mice after an ip glucose load. A and C, Among males, there were no differences in glucose tolerance [repeated-measures (RM) ANCOVA, ANCOVA]. B, DES females exhibited impaired glucose tolerance compared with control and BPA mice [RM ANCOVA followed by Tukey post hoc tests: P (maternal treatment) <0.01, _P_ (litter size) >0.05, P (DES vs. control) <0.01, _P_ (DES _vs_. BPA) <0.0.05]. _Symbols_ indicate significant Tukey _post hoc_ comparisons (*, _P_ < 0.05; **, _P_ < 0.01; ***, _P_ < 0.001) _vs_. control. D, DES females exhibited impaired glucose tolerance as compared by area under the curve (AUC) [ANCOVA followed by Tukey _post hoc_ tests: _P_ (maternal treatment) <0.05, _P_ (litter size) >0.05, P (DES vs. control <0.05)].
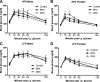
Figure 5
Growth on HFD vs. LFD from 9–14 wk of age. Cumulative caloric intake was increased in male and female mice eating a HFD vs. LFD. A, Among males, there was no effect of maternal treatment on caloric intake [ANCOVA: P (diet) <0.001, _P_ (maternal treatment) >0.05, P (litter size) >0.05]. B, Among females, control mice ate fewer calories compared with either BPA or DES mice [ANCOVA followed by Tukey post hoc tests: P (diet) <0.0001, _P_ (maternal treatment) <0.01, _P_ (control _vs_. BPA) <0.05, _P_ (control _vs_. DES) <0.05, _P_ (litter size) <0.05]. Male mice gained more weight while eating a HFD (C) _vs_. LFD (E). DES males tended to be lighter than either control or BPA males, although this difference did not reach statistical significance [repeated-measures (RM) ANCOVA followed by Tukey _post hoc_ tests: _P_ (maternal treatment) <0.05, _P_ (diet × time) <0.000001, _P_ (maternal treatment × time) <0.0001, _P_ (DES _vs_. control) <0.058, _P_ (DES _vs_. BPA) <0.058, _P_ (litter size) >0.05]. Female mice gained more weight while eating a HFD (D) vs. LFD (F). There were no differences in body weight among the groups [RM ANCOVA: P (maternal treatment) >0.05, P (diet × time) <0.001, P (litter size) <0.05, P (litter size × time) <0.05]. *, P < 0.05.
Figure 6
Body composition after 5 wk of HFD vs. LFD feeding. A, Among 14-wk-old males, HFD-fed mice had more body fat than LFD-fed mice. Control males also had more body fat than DES males [ANCOVA followed by Tukey post hoc tests: P (diet) <0.001, _P_ (maternal treatment) <0.05, _P_ (control _vs_. DES) <0.05, _P_ (litter size) >0.05]. B, Control females had more body fat than either BPA or DES females [ANCOVA followed by Tukey post hoc tests: P (maternal treatment) <0.01, _P_ (control _vs_. BPA) <0.01, _P_ (control _vs_. DES) <0.05, _P_ (litter size) >0.05]. There were no differences in lean mass among the groups in either males (C) or females (D) (ANCOVA). However, litter size did significantly predict lean body mass in 14-wk-old females (P < 0.05). *, P < 0.05; **, P < 0.01.
Figure 7
Blood glucose in 15-wk-old male and female mice after an ip glucose load. Among males, there were no differences in glucose tolerance regardless of whether the mice ate a HFD (A) or LFD (C) [repeated-measures (RM) ANCOVA]. B, Among females eating HFD, DES females exhibited impaired glucose tolerance [RM ANCOVA: P (maternal treatment) <0.05, P (time × maternal treatment) <0.01; symbols indicate significant Tukey post hoc comparisons (*, P < 0.05; **, P < 0.01) vs. the control females]. D, Among females eating LFD, there were no differences in glucose tolerance (RM ANCOVA).
Figure 8
Area under the curve (AUC) for 15-wk-old male and female mice after an ip glucose load. Among males, there were no differences in AUC regardless of whether the mice ate a HFD (A) or LFD (C) (ANCOVA). B, Among females eating a HFD, DES females had significantly higher AUC compared with BPA females [ANCOVA followed by Tukey post hoc comparisons: P (maternal treatment) <0.05, P (DES vs. BPA) <0.01]. D, Among females eating a LFD, there were no significant differences in AUC (ANCOVA). **, P < 0.01.
Similar articles
- Programming of metabolic effects in C57BL/6JxFVB mice by exposure to bisphenol A during gestation and lactation.
van Esterik JC, Dollé ME, Lamoree MH, van Leeuwen SP, Hamers T, Legler J, van der Ven LT. van Esterik JC, et al. Toxicology. 2014 Jul 3;321:40-52. doi: 10.1016/j.tox.2014.04.001. Epub 2014 Apr 13. Toxicology. 2014. PMID: 24726836 - Organizational effects of perinatal exposure to bisphenol-A and diethylstilbestrol on arcuate nucleus circuitry controlling food intake and energy expenditure in male and female CD-1 mice.
Mackay H, Patterson ZR, Khazall R, Patel S, Tsirlin D, Abizaid A. Mackay H, et al. Endocrinology. 2013 Apr;154(4):1465-75. doi: 10.1210/en.2012-2044. Epub 2013 Mar 14. Endocrinology. 2013. PMID: 23493373 - Transgenerational effect of parental obesity and chronic parental bisphenol A exposure on hormonal profile and reproductive organs of preadolescent Wistar rats of F1 generation: A one-generation study.
Dabeer S, Afjal MA, Ahmad S, Fatima M, Habib H, Parvez S, Raisuddin S. Dabeer S, et al. Hum Exp Toxicol. 2020 Jan;39(1):59-76. doi: 10.1177/0960327119873017. Epub 2019 Sep 11. Hum Exp Toxicol. 2020. PMID: 31510804 - Bisphenol A, obesity, and type 2 diabetes mellitus: genuine concern or unnecessary preoccupation?
Mirmira P, Evans-Molina C. Mirmira P, et al. Transl Res. 2014 Jul;164(1):13-21. doi: 10.1016/j.trsl.2014.03.003. Epub 2014 Mar 13. Transl Res. 2014. PMID: 24686036 Free PMC article. Review. - Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure.
Welshons WV, Nagel SC, vom Saal FS. Welshons WV, et al. Endocrinology. 2006 Jun;147(6 Suppl):S56-69. doi: 10.1210/en.2005-1159. Epub 2006 May 11. Endocrinology. 2006. PMID: 16690810 Review.
Cited by
- Urinary bisphenol A and obesity: NHANES 2003-2006.
Carwile JL, Michels KB. Carwile JL, et al. Environ Res. 2011 Aug;111(6):825-30. doi: 10.1016/j.envres.2011.05.014. Epub 2011 Jun 14. Environ Res. 2011. PMID: 21676388 Free PMC article. - Endocrine disruptors in the etiology of type 2 diabetes mellitus.
Alonso-Magdalena P, Quesada I, Nadal A. Alonso-Magdalena P, et al. Nat Rev Endocrinol. 2011 Jun;7(6):346-53. doi: 10.1038/nrendo.2011.56. Epub 2011 Apr 5. Nat Rev Endocrinol. 2011. PMID: 21467970 Review. - Bisphenol A diglycidyl ether induces adipogenic differentiation of multipotent stromal stem cells through a peroxisome proliferator-activated receptor gamma-independent mechanism.
Chamorro-García R, Kirchner S, Li X, Janesick A, Casey SC, Chow C, Blumberg B. Chamorro-García R, et al. Environ Health Perspect. 2012 Jul;120(7):984-9. doi: 10.1289/ehp.1205063. Epub 2012 May 25. Environ Health Perspect. 2012. PMID: 22763116 Free PMC article. - Maternal bisphenol a exposure during pregnancy and its association with adipokines in Mexican-American children.
Volberg V, Harley K, Calafat AM, Davé V, McFadden J, Eskenazi B, Holland N. Volberg V, et al. Environ Mol Mutagen. 2013 Oct;54(8):621-8. doi: 10.1002/em.21803. Epub 2013 Aug 1. Environ Mol Mutagen. 2013. PMID: 23908009 Free PMC article. - Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A.
Hengstler JG, Foth H, Gebel T, Kramer PJ, Lilienblum W, Schweinfurth H, Völkel W, Wollin KM, Gundert-Remy U. Hengstler JG, et al. Crit Rev Toxicol. 2011 Apr;41(4):263-91. doi: 10.3109/10408444.2011.558487. Crit Rev Toxicol. 2011. PMID: 21438738 Free PMC article. Review.
References
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV 2007 Human exposure to bisphenol A (BPA). Reprod Toxicol 24:139–177 - PubMed
- Chapin R, Adams J, Boekelheide K, Gray L, Hayward S, Lees P, McIntyre B, Portier K, Schnorr T, Selevan S, Vandenbergh J, Woskie S 2008 NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B 83:157–395 - PubMed
- Center for the Evaluation of Risks to Human Reproduction 2008 NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. Pittsburgh: National Toxicology Program, Department of Health and Human Services - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK073505/DK/NIDDK NIH HHS/United States
- R01 DK073505/DK/NIDDK NIH HHS/United States
- DK054890/DK/NIDDK NIH HHS/United States
- DK082173/DK/NIDDK NIH HHS/United States
- R01 DK054890/DK/NIDDK NIH HHS/United States
- F32 DK082173/DK/NIDDK NIH HHS/United States
- R01 DK078201/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous