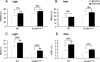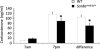Dysfunction of the Scn8a voltage-gated sodium channel alters sleep architecture, reduces diurnal corticosterone levels, and enhances spatial memory - PubMed (original) (raw)
Dysfunction of the Scn8a voltage-gated sodium channel alters sleep architecture, reduces diurnal corticosterone levels, and enhances spatial memory
Ligia A Papale et al. J Biol Chem. 2010.
Abstract
Voltage-gated sodium channels (VGSCs) are responsible for the initiation and propagation of transient depolarizing currents and play a critical role in the electrical signaling between neurons. A null mutation in the VGSC gene SCN8A, which encodes the transmembrane protein Na(v)1.6, was identified previously in a human family. Heterozygous mutation carriers displayed a range of phenotypes, including ataxia, cognitive deficits, and emotional instability. A possible role for SCN8A was also proposed in studies examining the genetic basis of attempted suicide and bipolar disorder. In addition, mice with a Scn8a loss-of-function mutation (Scn8a(med-Tg/+)) show altered anxiety and depression-like phenotypes. Because psychiatric abnormalities are often associated with altered sleep and hormonal patterns, we evaluated heterozygous Scn8a(med-jo/+) mutants for alterations in sleep-wake architecture, diurnal corticosterone levels, and behavior. Compared with their wild-type littermates, Scn8a(med-jo/+) mutants experience more non-rapid eye movement (non-REM) sleep, a chronic impairment of REM sleep generation and quantity, and a lowered and flattened diurnal rhythm of corticosterone levels. No robust differences were observed between mutants and wild-type littermates in locomotor activity or in behavioral paradigms that evaluate anxiety or depression-like phenotypes; however, Scn8a(med-jo/+) mutants did show enhanced spatial memory. This study extends the spectrum of phenotypes associated with mutations in Scn8a and suggests a novel role for altered sodium channel function in human sleep disorders.
Figures
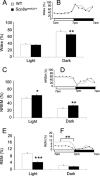
FIGURE 1.
The Scn8a med-jo/+ mutation reduces wakefulness, enhances NREM sleep, and reduces REM sleep amounts. Percentage of time spent in wakefulness (A and B), NREM sleep (C and D), and REM sleep (E and F) during the base-line recording period. Data were averaged over light and dark phases (A, C, and E). The circadian variation of the sleep-wake cycle was obtained by dividing the light and dark periods into 2-h intervals (B, D, and F). Black bars and squares, Scn8a med-jo/+; white bars and squares, WT. Values are presented as mean ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.0001; rANOVA followed by Tukey's post hoc test.
FIGURE 2.
Effect of 6 h of total SD in Scn8a med-jo/+ and WT littermates. Comparison of the amount of time spent in NREM sleep (A and B) and REM sleep (C and D), during 18 h of the recovery period with the equivalent time period during base-line recording. The recovery period comprised 6 h of the light period (1 p.m. to 7 p.m.) (A and C) and 12 h of the dark period (B and D). White bars, base-line period; black bars, recovery period. Values are presented as mean ± S.E. *, p < 0.05; **, p < 0.001; rANOVA followed by Tukey's post hoc test.
FIGURE 3.
Altered diurnal CORT rhythm in Scn8a med-jo/+ mutants. CORT levels are shown at the beginning of the light phase (7 a.m.) and at the beginning of the dark phase (7 p.m.). The difference in the magnitude of CORT levels between the 7 a.m. and 7 p.m. samples is also shown. White bars, WT; black bars, Scn8a med-jo/+. Error bars, mean ± S.E. *, p < 0.01, rANOVA followed by Tukey's post hoc test, for 7 p.m. CORT levels. *, p < 0.01, t test, for difference between 7 p.m. and 7 a.m. CORT levels.
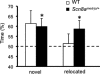
FIGURE 4.
Scn8a_med-jo_/+ mutants show modest enhancement in spatial memory. Shown is a comparison of the percentage of time spent exploring the novel and relocated objects. A value of 50% represents chance performance. White bars, WT; black bars, Scn8a med-jo/+. Error bars, mean ± S.E. *, p < 0.05, one-tailed t test.
Similar articles
- The voltage-gated sodium channel Scn8a is a genetic modifier of severe myoclonic epilepsy of infancy.
Martin MS, Tang B, Papale LA, Yu FH, Catterall WA, Escayg A. Martin MS, et al. Hum Mol Genet. 2007 Dec 1;16(23):2892-9. doi: 10.1093/hmg/ddm248. Epub 2007 Sep 19. Hum Mol Genet. 2007. PMID: 17881658 - Heterozygous mutations of the voltage-gated sodium channel SCN8A are associated with spike-wave discharges and absence epilepsy in mice.
Papale LA, Beyer B, Jones JM, Sharkey LM, Tufik S, Epstein M, Letts VA, Meisler MH, Frankel WN, Escayg A. Papale LA, et al. Hum Mol Genet. 2009 May 1;18(9):1633-41. doi: 10.1093/hmg/ddp081. Epub 2009 Mar 2. Hum Mol Genet. 2009. PMID: 19254928 Free PMC article. - Exaggerated emotional behavior in mice heterozygous null for the sodium channel Scn8a (Nav1.6).
McKinney BC, Chow CY, Meisler MH, Murphy GG. McKinney BC, et al. Genes Brain Behav. 2008 Aug;7(6):629-38. doi: 10.1111/j.1601-183X.2008.00399.x. Epub 2008 Mar 21. Genes Brain Behav. 2008. PMID: 18363861 Free PMC article. - Sodium channels and neurological disease: insights from Scn8a mutations in the mouse.
Meisler MH, Kearney J, Escayg A, MacDonald BT, Sprunger LK. Meisler MH, et al. Neuroscientist. 2001 Apr;7(2):136-45. doi: 10.1177/107385840100700208. Neuroscientist. 2001. PMID: 11496924 Review. - Cerebellum-related characteristics of Scn8a-mutant mice.
Chen K, Godfrey DA, Ilyas O, Xu J, Preston TW. Chen K, et al. Cerebellum. 2009 Sep;8(3):192-201. doi: 10.1007/s12311-009-0110-z. Epub 2009 May 8. Cerebellum. 2009. PMID: 19424768 Review.
Cited by
- Voltage Gated Ion Channels and Sleep.
Zhang Y, Wu J, Zheng Y, Xu Y, Yu Z, Ping Y. Zhang Y, et al. J Membr Biol. 2024 Oct 1. doi: 10.1007/s00232-024-00325-0. Online ahead of print. J Membr Biol. 2024. PMID: 39354150 Review. - Prefrontal PV interneurons facilitate attention and are linked to attentional dysfunction in a mouse model of absence epilepsy.
Ferguson B, Glick C, Huguenard JR. Ferguson B, et al. Elife. 2023 Apr 4;12:e78349. doi: 10.7554/eLife.78349. Elife. 2023. PMID: 37014118 Free PMC article. - Critical periods and Autism Spectrum Disorders, a role for sleep.
Medina E, Peterson S, Ford K, Singletary K, Peixoto L. Medina E, et al. Neurobiol Sleep Circadian Rhythms. 2022 Dec 20;14:100088. doi: 10.1016/j.nbscr.2022.100088. eCollection 2023 May. Neurobiol Sleep Circadian Rhythms. 2022. PMID: 36632570 Free PMC article. - Deficiency of autism-related Scn2a gene in mice disrupts sleep patterns and circadian rhythms.
Ma Z, Eaton M, Liu Y, Zhang J, Chen X, Tu X, Shi Y, Que Z, Wettschurack K, Zhang Z, Shi R, Chen Y, Kimbrough A, Lanman NA, Schust L, Huang Z, Yang Y. Ma Z, et al. Neurobiol Dis. 2022 Jun 15;168:105690. doi: 10.1016/j.nbd.2022.105690. Epub 2022 Mar 14. Neurobiol Dis. 2022. PMID: 35301122 Free PMC article. - Sleep Disorders in Children With Autism Spectrum Disorder: Insights From Animal Models, Especially Non-human Primate Model.
Feng S, Huang H, Wang N, Wei Y, Liu Y, Qin D. Feng S, et al. Front Behav Neurosci. 2021 May 20;15:673372. doi: 10.3389/fnbeh.2021.673372. eCollection 2021. Front Behav Neurosci. 2021. PMID: 34093147 Free PMC article. Review.
References
- Boiko T., Rasband M. N., Levinson S. R., Caldwell J. H., Mandel G., Trimmer J. S., Matthews G. (2001) Neuron 30, 91–104 - PubMed
- Smith M. R., Goldin A. L. (1999) Neuroreport 10, 3027–3031 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS060659/NS/NINDS NIH HHS/United States
- NS065187/NS/NINDS NIH HHS/United States
- U54 NS060659/NS/NINDS NIH HHS/United States
- NS046484/NS/NINDS NIH HHS/United States
- R01 NS065187/NS/NINDS NIH HHS/United States
- R01 NS046484/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous