The Beclin 1-VPS34 complex--at the crossroads of autophagy and beyond - PubMed (original) (raw)
Review
The Beclin 1-VPS34 complex--at the crossroads of autophagy and beyond
Sarah F Funderburk et al. Trends Cell Biol. 2010 Jun.
Abstract
An increasing body of research on autophagy provides overwhelming evidence for its connection to diverse biological functions and human diseases. Beclin 1, the first mammalian autophagy protein to be described, appears to act as a nexus point between autophagy, endosomal, and perhaps also cell death pathways. Beclin 1 performs these roles as part of a core complex that contains vacuolar sorting protein 34 (VPS34), a class III phosphatidylinositol-3 kinase. The precise mechanism of Beclin 1-mediated regulation of these cellular functions is unclear, but substantial progress has recently been made in identifying new players and their functions in Beclin 1-VSP34 complexes. Here we review emerging studies that are beginning to unveil the physiological functions of Beclin 1-VPS34 in the central control of autophagic activity and other trafficking events through the formation of distinct Beclin 1-VPS34 protein complexes.
(c) 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Note: The authors declare No conflicts of interest.
Figures
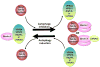
Figure 1. Multiple Beclin 1-VPS34 complexes and their distinct functions
(A) An illustration of the multilayered Beclin 1 protein complexes: a core complex Beclin 1-VPS34-VPS15; stable binding partners Atg14L, UVRAG, and Rubicon; unstable or transient binding partners including Bcl-2 homologs, Bif-1, Ambra 1, VMP1, nPIST, Rab5, ICP 34.5, and inositol 1,4,5-triphosphate receptor (IP3R). Among the unstable binding partners, Bif-1 interacts with UVRAG, and IP3R interacts with Bcl-2. (B) In yeast two Atg6-Vps34 complexes, I and II, regulate autophagy and vacuolar protein sorting, respectively. In mammals, multiple Beclin 1 complexes act in different stages of autophagy and perhaps also endocytic trafficking. Atg14L associates with the core Beclin 1-VPS34-VPS15 complex to function in the biosynthesis of autophagosomes, and UVRAG potentially associates with the same core complex to also influence autophagosome formation. However, UVRAG has an additional role in autophagosome maturation in a complex with C-Vps and the Rab7 GTPase that is independent of Beclin 1. Rubicon inhibits autophagosome maturation in a possibly Beclin 1-independent manner. Rubicon could sequester Rab proteins such as Rab 7 through its RUN domain to inhibit this stage of autophagy. The binding of Rubicon to the Beclin 1 core complex though UVRAG may aid in neutralizing such a negative function.
Figure 2. Dynamic regulation of Beclin 1-VPS34 complex formation and autophagy by Bcl-2 family members and Beclin 1 dimerization
Beclin 1-VPS34-VPS15 forms pro-autophagic complexes with Atg14L or UVRAG. Binding of Bcl-2 and Beclin 1 is known to inhibit autophagy by possibly disrupting VPS34 binding; but Bcl-2 binding may also displace other stable interacting partners such as Atg14L (or UVRAG), resulting in autophagy inhibition. Additionally, the anti-autophagic binding of Bcl-2 promotes Beclin 1 homodimerization, which prevents heterodimerization with Atg14L or UVRAG.
Similar articles
- Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG.
Itakura E, Kishi C, Inoue K, Mizushima N. Itakura E, et al. Mol Biol Cell. 2008 Dec;19(12):5360-72. doi: 10.1091/mbc.e08-01-0080. Epub 2008 Oct 8. Mol Biol Cell. 2008. PMID: 18843052 Free PMC article. - Nrbf2 protein suppresses autophagy by modulating Atg14L protein-containing Beclin 1-Vps34 complex architecture and reducing intracellular phosphatidylinositol-3 phosphate levels.
Zhong Y, Morris DH, Jin L, Patel MS, Karunakaran SK, Fu YJ, Matuszak EA, Weiss HL, Chait BT, Wang QJ. Zhong Y, et al. J Biol Chem. 2014 Sep 19;289(38):26021-26037. doi: 10.1074/jbc.M114.561134. Epub 2014 Aug 1. J Biol Chem. 2014. PMID: 25086043 Free PMC article. - Beclin 1 is required for neuron viability and regulates endosome pathways via the UVRAG-VPS34 complex.
McKnight NC, Zhong Y, Wold MS, Gong S, Phillips GR, Dou Z, Zhao Y, Heintz N, Zong WX, Yue Z. McKnight NC, et al. PLoS Genet. 2014 Oct 2;10(10):e1004626. doi: 10.1371/journal.pgen.1004626. eCollection 2014 Oct. PLoS Genet. 2014. PMID: 25275521 Free PMC article. - The Beclin 1 network regulates autophagy and apoptosis.
Kang R, Zeh HJ, Lotze MT, Tang D. Kang R, et al. Cell Death Differ. 2011 Apr;18(4):571-80. doi: 10.1038/cdd.2010.191. Epub 2011 Feb 11. Cell Death Differ. 2011. PMID: 21311563 Free PMC article. Review. - Ubiquitination and phosphorylation of Beclin 1 and its binding partners: Tuning class III phosphatidylinositol 3-kinase activity and tumor suppression.
Abrahamsen H, Stenmark H, Platta HW. Abrahamsen H, et al. FEBS Lett. 2012 Jun 4;586(11):1584-91. doi: 10.1016/j.febslet.2012.04.046. Epub 2012 May 3. FEBS Lett. 2012. PMID: 22673570 Review.
Cited by
- Genome-wide CRISPR/Cas9 screen identifies SLC39A9 and PIK3C3 as crucial entry factors for Ebola virus infection.
Gong M, Peng C, Yang C, Wang Z, Qian H, Hu X, Zhou P, Shan C, Ding Q. Gong M, et al. PLoS Pathog. 2024 Aug 22;20(8):e1012444. doi: 10.1371/journal.ppat.1012444. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39173055 Free PMC article. - A conserved myotubularin-related phosphatase regulates autophagy by maintaining autophagic flux.
Allen EA, Amato C, Fortier TM, Velentzas P, Wood W, Baehrecke EH. Allen EA, et al. J Cell Biol. 2020 Nov 2;219(11):e201909073. doi: 10.1083/jcb.201909073. J Cell Biol. 2020. PMID: 32915229 Free PMC article. - SLC9A3R1 stimulates autophagy via BECN1 stabilization in breast cancer cells.
Liu H, Ma Y, He HW, Wang JP, Jiang JD, Shao RG. Liu H, et al. Autophagy. 2015;11(12):2323-34. doi: 10.1080/15548627.2015.1074372. Autophagy. 2015. PMID: 26218645 Free PMC article. - Autophagy Roles in Genome Maintenance.
Ambrosio S, Majello B. Ambrosio S, et al. Cancers (Basel). 2020 Jul 4;12(7):1793. doi: 10.3390/cancers12071793. Cancers (Basel). 2020. PMID: 32635505 Free PMC article. Review. - Major vault protein in cardiac and smooth muscle.
Shults NV, Das D, Suzuki YJ. Shults NV, et al. Receptors Clin Investig. 2016;3(2):e1310. Epub 2016 May 23. Receptors Clin Investig. 2016. PMID: 27275002 Free PMC article.
References
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. - PubMed
- Liang XH, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources