Detection of transient interchain interactions in the intrinsically disordered protein alpha-synuclein by NMR paramagnetic relaxation enhancement - PubMed (original) (raw)
Detection of transient interchain interactions in the intrinsically disordered protein alpha-synuclein by NMR paramagnetic relaxation enhancement
Kuen-Phon Wu et al. J Am Chem Soc. 2010.
Abstract
NMR paramagnetic relaxation enhancement experiments were applied to the intrinsically disordered protein alpha-synuclein, the primary protein in Parkinson's disease, to directly characterize transient intermolecular complexes at neutral and low pH. At neutral pH, we observed weak N- to C-terminal interchain contacts driven by electrostatic interactions, while at low pH, the C- to C-terminal interchain interactions are significantly stronger and driven by hydrophobic contacts. Characterization of these first interchain interactions will provide fundamental insight into the mechanism of amyloid formation.
Figures
Figure 1
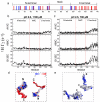
Transient encounter complexes in the intrinsically disordered protein αSyn. The distribution of positively and negatively charged residues is shown with blue and red bars respectively (a). Inter-chain NMR PRE profiles for intrinsically disordered αSyn at pH 6.0 (b) and pH 2.5 (c). 1H Γ2 values with MTSL labels at A19C, A90C and G132C are shown for both sets of conditions. Models that illustrate the inter-chain interactions observed from the PRE experiments at neutral (d) and low pH (e). Monomer conformations were selected from the REMD ensemble generated at neutral and low pH in ref. and are shown as electrostatic surface potentials. The surface potential was calculated by Delphi and the color gradient from red to blue indicates surface potential from −2 to 2 kT/e.
Similar articles
- Structural reorganization of alpha-synuclein at low pH observed by NMR and REMD simulations.
Wu KP, Weinstock DS, Narayanan C, Levy RM, Baum J. Wu KP, et al. J Mol Biol. 2009 Aug 28;391(4):784-96. doi: 10.1016/j.jmb.2009.06.063. Epub 2009 Jul 1. J Mol Biol. 2009. PMID: 19576220 Free PMC article. - Characterization of conformational and dynamic properties of natively unfolded human and mouse alpha-synuclein ensembles by NMR: implication for aggregation.
Wu KP, Kim S, Fela DA, Baum J. Wu KP, et al. J Mol Biol. 2008 May 16;378(5):1104-15. doi: 10.1016/j.jmb.2008.03.017. Epub 2008 Mar 18. J Mol Biol. 2008. PMID: 18423664 Free PMC article. - Intermolecular Paramagnetic Relaxation Enhancement (PRE) Studies of Transient Complexes in Intrinsically Disordered Proteins.
Janowska MK, Baum J. Janowska MK, et al. Methods Mol Biol. 2016;1345:45-53. doi: 10.1007/978-1-4939-2978-8_3. Methods Mol Biol. 2016. PMID: 26453204 - Bacterial in-cell NMR of human α-synuclein: a disordered monomer by nature?
Binolfi A, Theillet FX, Selenko P. Binolfi A, et al. Biochem Soc Trans. 2012 Oct;40(5):950-4. doi: 10.1042/BST20120096. Biochem Soc Trans. 2012. PMID: 22988846 Review. - The Cellular Environment Affects Monomeric α-Synuclein Structure.
Stephens AD, Zacharopoulou M, Kaminski Schierle GS. Stephens AD, et al. Trends Biochem Sci. 2019 May;44(5):453-466. doi: 10.1016/j.tibs.2018.11.005. Epub 2018 Dec 7. Trends Biochem Sci. 2019. PMID: 30527975 Review.
Cited by
- Evidence for Intramolecular Antiparallel Beta-Sheet Structure in Alpha-Synuclein Fibrils from a Combination of Two-Dimensional Infrared Spectroscopy and Atomic Force Microscopy.
Roeters SJ, Iyer A, Pletikapić G, Kogan V, Subramaniam V, Woutersen S. Roeters SJ, et al. Sci Rep. 2017 Jan 23;7:41051. doi: 10.1038/srep41051. Sci Rep. 2017. PMID: 28112214 Free PMC article. - Visualizing and trapping transient oligomers in amyloid assembly pathways.
Cawood EE, Karamanos TK, Wilson AJ, Radford SE. Cawood EE, et al. Biophys Chem. 2021 Jan;268:106505. doi: 10.1016/j.bpc.2020.106505. Epub 2020 Nov 10. Biophys Chem. 2021. PMID: 33220582 Free PMC article. Review. - The quiet renaissance of protein nuclear magnetic resonance.
Barrett PJ, Chen J, Cho MK, Kim JH, Lu Z, Mathew S, Peng D, Song Y, Van Horn WD, Zhuang T, Sönnichsen FD, Sanders CR. Barrett PJ, et al. Biochemistry. 2013 Feb 26;52(8):1303-20. doi: 10.1021/bi4000436. Epub 2013 Feb 12. Biochemistry. 2013. PMID: 23368985 Free PMC article. Review. - Exploring the accessible conformations of N-terminal acetylated α-synuclein.
Moriarty GM, Janowska MK, Kang L, Baum J. Moriarty GM, et al. FEBS Lett. 2013 Apr 17;587(8):1128-38. doi: 10.1016/j.febslet.2013.02.049. Epub 2013 Mar 13. FEBS Lett. 2013. PMID: 23499431 Free PMC article. Review. - Paramagnetic Relaxation Enhancement for Detecting and Characterizing Self-Associations of Intrinsically Disordered Proteins.
Johnson CN, Libich DS. Johnson CN, et al. J Vis Exp. 2021 Sep 23;(175):10.3791/63057. doi: 10.3791/63057. J Vis Exp. 2021. PMID: 34633390 Free PMC article.
References
- Chiti F, Dobson CM. Annu Rev Biochem. 2006;75:333–66. - PubMed
- Mittag T, Forman-Kay JD. Curr. Opinion Struct. Biol. 2007;17:3–14. - PubMed
- Wright PE, Dyson HJ. Curr. Opinion Struct. Biol. 2009;19:31–38. - PMC - PubMed
- Eliezer D. Curr. Opinion Struct. Biol. 2009;19:23–30. - PMC - PubMed
- Wu K-P, Kim S, Fela DA, Baum J. J Mol Biol. 2008;378:1104–15. - PMC - PubMed
- Dedmon MM, Lindorff-Larsen K, Christodoulou J, Vendruscolo M, Dobson CM. J. Am. Chem. Soc. 2005;127:476–477. - PubMed
- Bertoncini CW, Jung YS, Fernandez CO, Hoyer W, Griesinger C, Jovin TM, Zweckstetter M. Proc Natl Acad Sci U S A. 2005;102:1430–5. - PMC - PubMed
- Uversky VN, Gillespie JR, Fink AL. Proteins. 2000;41:415–27. - PubMed
- Uversky VN, Li J, Fink AL. J Biol Chem. 2001;276:10737–44. - PubMed
- Wu K-P, Weinstock DS, Narayanan C, Levy RM, Baum J. J Mol Biol. 2009;391:784–796. - PMC - PubMed
- Cho MK, Nodet G, Kim HY, Jensen MR, Bernado P, Fernandez CO, Becker S, Blackledge M, Zweckstetter M. Protein Sci. 2009;18:1840–6. - PMC - PubMed
- McClendon S, Rospigliosi CC, Eliezer D. Protein Sci. 2009;18:1531–40. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM045302-17/GM/NIGMS NIH HHS/United States
- R01 GM087012-02/GM/NIGMS NIH HHS/United States
- R01 GM045302/GM/NIGMS NIH HHS/United States
- GM087012/GM/NIGMS NIH HHS/United States
- R01 GM087012/GM/NIGMS NIH HHS/United States
- GM45302/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources