Publication bias in reports of animal stroke studies leads to major overstatement of efficacy - PubMed (original) (raw)
Publication bias in reports of animal stroke studies leads to major overstatement of efficacy
Emily S Sena et al. PLoS Biol. 2010.
Abstract
The consolidation of scientific knowledge proceeds through the interpretation and then distillation of data presented in research reports, first in review articles and then in textbooks and undergraduate courses, until truths become accepted as such both amongst "experts" and in the public understanding. Where data are collected but remain unpublished, they cannot contribute to this distillation of knowledge. If these unpublished data differ substantially from published work, conclusions may not reflect adequately the underlying biological effects being described. The existence and any impact of such "publication bias" in the laboratory sciences have not been described. Using the CAMARADES (Collaborative Approach to Meta-analysis and Review of Animal Data in Experimental Studies) database we identified 16 systematic reviews of interventions tested in animal studies of acute ischaemic stroke involving 525 unique publications. Only ten publications (2%) reported no significant effects on infarct volume and only six (1.2%) did not report at least one significant finding. Egger regression and trim-and-fill analysis suggested that publication bias was highly prevalent (present in the literature for 16 and ten interventions, respectively) in animal studies modelling stroke. Trim-and-fill analysis suggested that publication bias might account for around one-third of the efficacy reported in systematic reviews, with reported efficacy falling from 31.3% to 23.8% after adjustment for publication bias. We estimate that a further 214 experiments (in addition to the 1,359 identified through rigorous systematic review; non publication rate 14%) have been conducted but not reported. It is probable that publication bias has an important impact in other animal disease models, and more broadly in the life sciences.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Figure 1. QUOROM chart of fate of 71 publications identified in systematic search for studies reporting the quantitative impact of publication bias in reports of animal experiments modelling human disease.
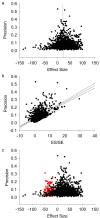
Figure 2. Example funnel plots, Egger regressions, and trim-and-fill plots.
Data from meta-analyses of hypothermia (a,e,i), tPA (b,f,j), stem cells (c,g,k), and growth factors (d,h,l). (a–d) Funnel plots showing precision plotted against effect size. In the absence of publication bias the points should resemble an inverted funnel. (e–h) Egger regression showing precision plotted against the standardised effect size. In the absence of publication bias the regression line should pass through the origin. (i–l) Funnel plots showing the data from (a) to (d) in black, and the additional missing studies imputed by trim-and-fill in red.
Figure 3. Plots describing the complete dataset.
Funnel plot (a), Egger regression (b), and trim-and-fill plots (c). See Figure 1 legend for details.
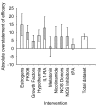
Figure 4. Absolute overstatement of efficacy for the ten interventions identified through trim-and-fill as showing significant publication bias.
The vertical error bars represent the 95% confidence intervals of the estimate. The width of each column reflects the log of the number of contributing experiments.
Similar articles
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - Publication Bias and Nonreporting Found in Majority of Systematic Reviews and Meta-analyses in Anesthesiology Journals.
Hedin RJ, Umberham BA, Detweiler BN, Kollmorgen L, Vassar M. Hedin RJ, et al. Anesth Analg. 2016 Oct;123(4):1018-25. doi: 10.1213/ANE.0000000000001452. Anesth Analg. 2016. PMID: 27537925 Review. - Publication bias in dermatology systematic reviews and meta-analyses.
Atakpo P, Vassar M. Atakpo P, et al. J Dermatol Sci. 2016 May;82(2):69-74. doi: 10.1016/j.jdermsci.2016.02.005. Epub 2016 Feb 24. J Dermatol Sci. 2016. PMID: 26925817 - The future of Cochrane Neonatal.
Soll RF, Ovelman C, McGuire W. Soll RF, et al. Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834 - Systematic review and meta-analysis of interventions tested in animal models of lacunar stroke.
Pedder H, Vesterinen HM, Macleod MR, Wardlaw JM. Pedder H, et al. Stroke. 2014 Feb;45(2):563-70. doi: 10.1161/STROKEAHA.113.003128. Epub 2014 Jan 2. Stroke. 2014. PMID: 24385271 Review.
Cited by
- Conflict of interest in spine research reporting.
Walcott BP, Sheth SA, Nahed BV, Coumans JV. Walcott BP, et al. PLoS One. 2012;7(8):e44327. doi: 10.1371/journal.pone.0044327. Epub 2012 Aug 31. PLoS One. 2012. PMID: 22952956 Free PMC article. - A Handful of Details to Ensure the Experimental Reproducibility on the FORCED Running Wheel in Rodents: A Systematic Review.
Garrigos D, Martínez-Morga M, Toval A, Kutsenko Y, Barreda A, Do Couto BR, Navarro-Mateu F, Ferran JL. Garrigos D, et al. Front Endocrinol (Lausanne). 2021 May 10;12:638261. doi: 10.3389/fendo.2021.638261. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34040580 Free PMC article. - The usefulness of systematic reviews of animal experiments for the design of preclinical and clinical studies.
de Vries RB, Wever KE, Avey MT, Stephens ML, Sena ES, Leenaars M. de Vries RB, et al. ILAR J. 2014;55(3):427-37. doi: 10.1093/ilar/ilu043. ILAR J. 2014. PMID: 25541545 Free PMC article. Review. - The 1027th target candidate in stroke: Will NADPH oxidase hold up?
Radermacher KA, Wingler K, Kleikers P, Altenhöfer S, Jr Hermans J, Kleinschnitz C, Hhw Schmidt H. Radermacher KA, et al. Exp Transl Stroke Med. 2012 May 24;4(1):11. doi: 10.1186/2040-7378-4-11. Exp Transl Stroke Med. 2012. PMID: 22625431 Free PMC article. - Importance of Systematic Reviews and Meta-analyses of Animal Studies: Challenges for Animal-to-Human Translation.
Bahadoran Z, Mirmiran P, Kashfi K, Ghasemi A. Bahadoran Z, et al. J Am Assoc Lab Anim Sci. 2020 Sep 1;59(5):469-477. doi: 10.30802/AALAS-JAALAS-19-000139. Epub 2020 Jul 29. J Am Assoc Lab Anim Sci. 2020. PMID: 32727637 Free PMC article.
References
- Oxman A. D, Guyatt G. H. The science of reviewing research. Ann N Y Acad Sci. 1993;703:125–33; discussion 133–4. 125–133. - PubMed
- Antman E. M, Lau J, Kupelnick B, Mosteller F, Chalmers T. C. A comparison of results of meta-analyses of randomized control trials and recommendations of clinical experts. Treatments for myocardial infarction. JAMA. 1992;268:240–248. - PubMed
- Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86:638–641.
- De Angelis C, Drazen J. M, Frizelle F. A, Haug C, Hoey J, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med. 2004;351:1250–1251. - PubMed
- Miettinen O. S. Up from ‘false positives’ in genetic-and other-epidemiology. Eur J Epidemiol. 2009;24:1–5. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical