Bevacizumab in recurrent high-grade pediatric gliomas - PubMed (original) (raw)
Bevacizumab in recurrent high-grade pediatric gliomas
Ashwatha Narayana et al. Neuro Oncol. 2010 Sep.
Abstract
Bevacizumab, a monoclonal antibody against vascular endothelial growth factor, has shown promise in treating recurrent adult high-grade glioma (HGG). However, there is very little data on recurrent or progressive pediatric HGG treated with bevacizumab. We report the results of a single institution experience using bevacizumab and irinotecan in children who relapsed or progressed following standard therapy. Twelve pediatric patients with recurrent or progressive HGG received bevacizumab at 10 mg/kg every 2 weeks with irinotecan at 125 mg/m(2). Magnetic resonance imaging (MRI) was performed prior to therapy and every 8 weeks subsequently. Ten patients had supratentorial HGG; 2 had DIPG. Radiological responses were defined according to MacDonald's criteria. Progression-free survival (PFS), overall survival (OS), and toxicities were analyzed. Ten (83.3%) patients tolerated bevacizumab without serious toxicity. Therapy was discontinued in 1 patient because of anaphylaxis. Another patient developed grade III delayed wound healing and deep vein thrombosis. Two patients (16.7%) experienced a partial response after the first MRI. No complete radiographic responses were seen. Stable disease was noted in 4 (33.3%) patients. The median PFS and OS were 2.25 and 6.25 months, respectively. A diffuse invasive recurrence pattern was noted in 5 (45.5%) patients. Treatment tolerance, toxicity, and recurrence profiles were comparable to adult HGG patients treated with bevacizumab. However, the radiological response rate, response duration, and survival appeared inferior in pediatric patients. Genetic differences in pediatric gliomas might account for this difference.
Figures
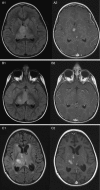
Fig. 1.
Four-year-old girl with recurrent anaplastic astrocytoma treated with bevacizumab and irinotecan. Image sets 1 and 2 represent T2/FLAIR and contrast-enhanced MRI sequences, respectively. Series A and B represent MRI images taken 2 weeks prior to treatment and 8 weeks after the start of treatment. Series C shows local tumor progression 5 months after the start of treatment.
Fig. 2.
Progression-free survival (PFS) and overall survival (OS).
Similar articles
- Treatment of children with recurrent high grade gliomas with a bevacizumab containing regimen.
Parekh C, Jubran R, Erdreich-Epstein A, Panigrahy A, Bluml S, Finlay J, Dhall G. Parekh C, et al. J Neurooncol. 2011 Jul;103(3):673-80. doi: 10.1007/s11060-010-0444-x. Epub 2010 Nov 1. J Neurooncol. 2011. PMID: 21038110 - FDG-PET predicts survival in recurrent high-grade gliomas treated with bevacizumab and irinotecan.
Colavolpe C, Chinot O, Metellus P, Mancini J, Barrie M, Bequet-Boucard C, Tabouret E, Mundler O, Figarella-Branger D, Guedj E. Colavolpe C, et al. Neuro Oncol. 2012 May;14(5):649-57. doi: 10.1093/neuonc/nos012. Epub 2012 Feb 29. Neuro Oncol. 2012. PMID: 22379188 Free PMC article. - Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma.
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Wagner M, Bigner DD, Friedman AH, Friedman HS. Vredenburgh JJ, et al. Clin Cancer Res. 2007 Feb 15;13(4):1253-9. doi: 10.1158/1078-0432.CCR-06-2309. Clin Cancer Res. 2007. PMID: 17317837 Clinical Trial. - Effects of bevacizumab plus irinotecan on response and survival in patients with recurrent malignant glioma: a systematic review and survival-gain analysis.
Xu T, Chen J, Lu Y, Wolff JE. Xu T, et al. BMC Cancer. 2010 Jun 2;10:252. doi: 10.1186/1471-2407-10-252. BMC Cancer. 2010. PMID: 20525214 Free PMC article. Review. - Emerging clinical principles on the use of bevacizumab for the treatment of malignant gliomas.
Chamberlain MC. Chamberlain MC. Cancer. 2010 Sep 1;116(17):3988-99. doi: 10.1002/cncr.25256. Cancer. 2010. PMID: 20564141 Review.
Cited by
- How I treat recurrent pediatric high-grade glioma (pHGG): a Europe-wide survey study.
Perwein T, Giese B, Nussbaumer G, von Bueren AO, van Buiren M, Benesch M, Kramm CM. Perwein T, et al. J Neurooncol. 2023 Feb;161(3):525-538. doi: 10.1007/s11060-023-04241-6. Epub 2023 Feb 1. J Neurooncol. 2023. PMID: 36720762 Free PMC article. - Pediatric Brain Tumors: Innovative Genomic Information Is Transforming the Diagnostic and Clinical Landscape.
Gajjar A, Bowers DC, Karajannis MA, Leary S, Witt H, Gottardo NG. Gajjar A, et al. J Clin Oncol. 2015 Sep 20;33(27):2986-98. doi: 10.1200/JCO.2014.59.9217. Epub 2015 Aug 24. J Clin Oncol. 2015. PMID: 26304884 Free PMC article. Review. - Management of high-grade gliomas in the pediatric patient: Past, present, and future.
Vanan MI, Eisenstat DD. Vanan MI, et al. Neurooncol Pract. 2014 Dec;1(4):145-157. doi: 10.1093/nop/npu022. Epub 2014 Sep 12. Neurooncol Pract. 2014. PMID: 26034626 Free PMC article. - Targeting tumor hypoxia and mitochondrial metabolism with anti-parasitic drugs to improve radiation response in high-grade gliomas.
Mudassar F, Shen H, O'Neill G, Hau E. Mudassar F, et al. J Exp Clin Cancer Res. 2020 Oct 7;39(1):208. doi: 10.1186/s13046-020-01724-6. J Exp Clin Cancer Res. 2020. PMID: 33028364 Free PMC article. Review. - Global Impact of Monoclonal Antibodies (mAbs) in Children: A Focus on Anti-GD2.
Larrosa C, Mora J, Cheung NK. Larrosa C, et al. Cancers (Basel). 2023 Jul 22;15(14):3729. doi: 10.3390/cancers15143729. Cancers (Basel). 2023. PMID: 37509390 Free PMC article. Review.
References
- Halperin E, Constine L, Tarbell N, Kun L. Pediatric Radiation Oncology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
- Mueller S, Chang S. Pediatric brain tumors: current treatment strategies and future therapeutic approaches. Neurotherapeutics. 2009;6(3):570–586. doi:10.1016/j.nurt.2009.04.006. - DOI - PMC - PubMed
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi:10.1056/NEJMoa032691. - DOI - PubMed
- Traina TA, Rugo HS, Dickler M. Bevacizumab for advanced breast cancer. Hematology/Oncology Clinics of North America. 2007;21(2):303–319. doi:10.1016/j.hoc.2007.03.006. - DOI - PubMed
- Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized Phase II Trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22(11):2184–2191. doi:10.1200/JCO.2004.11.022. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical