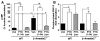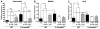A beta-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation - PubMed (original) (raw)
A beta-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation
Diane Gesty-Palmer et al. Sci Transl Med. 2009.
Erratum in
- Erratum for the Research Article "A β-Arrestin-Biased Agonist of the Parathyroid Hormone Receptor (PTH1R) Promotes Bone Formation Independent of G Protein Activation" by D. Gesty-Palmer et al.
[No authors listed] [No authors listed] Sci Transl Med. 2024 Aug 14;16(760):eadr6878. doi: 10.1126/scitranslmed.adr6878. Epub 2024 Aug 14. Sci Transl Med. 2024. PMID: 39141704 No abstract available.
Abstract
About 40% of the therapeutic agents in use today exert their effects through seven-transmembrane receptors (7TMRs). When activated by ligands, these receptors trigger two pathways that independently transduce signals to the cell: one through heterotrimeric GTP-binding proteins (G proteins) and one through beta-arrestins; so-called biased agonists can selectively activate these distinct pathways. Here, we investigate selective activation of these pathways through the use of a biased agonist for the type 1 parathyroid hormone (PTH)-PTH-related protein receptor (PTH1R), (D-Trp(12),Tyr(34))-PTH(7-34) (PTH-betaarr), which activates beta-arrestin but not classic G protein signaling. In mice, PTH-betaarr induces anabolic bone formation, as does the nonselective agonist PTH(1-34), which activates both mechanisms. In beta-arrestin2-null mice, the increase in bone mineral density evoked by PTH(1-34) is attenuated and that stimulated by PTH-betaarr is ablated. The beta-arrestin2-dependent pathway contributes primarily to trabecular bone formation and does not stimulate bone resorption. These results show that a biased agonist selective for the beta-arrestin pathway can elicit a response in vivo distinct from that elicited by nonselective agonists. Ligands with these properties may form the basis for improved 7TMR-directed pharmacologic agents with enhanced therapeutic specificity.
Conflict of interest statement
Competing interests: R.J.L. is a founder and member of the Scientific Advisory Board for Trevena, Inc., a company that discovers and develops novel G protein–coupled receptor–targeted medicines. R.J.L., D.G.-P., and L.M.L. have filed a patent related to the results reported in this paper.
Figures
Fig. 1
PTH-βarr stimulates β-arrestin–mediated ERK1/2 activation, independent of G protein signaling, in osteoblasts. (A) cAMP activation in response to PTH(1–34) and PTH-βarr stimulation of endogenously expressed PTH1R in POBs isolated from β-arrestin2−/− and WT C57BL/6 mice. cAMP values were normalized to 10 μM forskolin–induced concentrations (2.24 ± 0.2 μM). Data correspond to the mean ± SEM from four independent experiments. ***P < 0.001 compared with the vehicle-stimulated WT POBs. †††P < 0.001; ††P < 0.01 compared with the vehicle-stimulated β-arrestin2−/− POBs; direct comparisons were made with two-tailed unpaired t test. Veh, vehicle. (B) PTH(1–34) and PTH-βarr stimulated ERK1/2 activation in POBs isolated from β-arrestin2−/− and WT C57BL/6 mice. Values presented are the fold ERK1/2 phosphorylation over vehicle-stimulated controls. Data represent the mean ± SEM from four independent experiments. **P < 0.01 compared with the vehicle-stimulated WT POBs. ††P < 0.01 compared with the vehicle-stimulated β-arrestin2−/− POBs; direct comparisons were made with two-tailed unpaired t test.
Fig. 2
PTH-βarr increases lumbar spine BMD. (A and B) Lumbar spine and (C and D) femoral BMD of male WT and β-arrestin2−/− mice treated with vehicle (Veh), 1-34), or PTH-βarr was determined by dual-energy x-ray absorption. Mice were treated starting at 9 weeks of age. Data represent the mean percent change from baseline BMD ± SEM of measurements taken from at least seven male mice. *P < 0.05; **P < 0.01 compared with vehicle-treated WT mice. †P < 0.05, ††P < 0.01, †††P < 0.001 compared with vehicle-treated β-arrestin2−/− mice; significance was determined with one-way ANOVA with Bonferroni multiple comparisons post test.
Fig. 3
β-Arrestin2–dependent signaling contributes to increases in trabecular but not cortical bone. (A) Representative qCT of lumbar vertebrae isolated from male WT and β-arrestin2−/− mice treated for 8 weeks with vehicle, PTH(1–34), or PTH-βarr. Scale bar, 1.0 mm. Mice were treated starting at 9 weeks of age. qCT of the lumbar spine was used to determine the effects on (B) trabecular bone (Tb) volume fraction (BV/TV), (C) Tb thickness, and (D) Tb number. (E) Representative qCT of proximal tibia from male WT and β-arrestin2−/− mice treated for 8 weeks with vehicle, PTH(1–34), or PTH-βarr. Scale bar, 1.0 mm. qCT of proximal tibia was used to determine the effects on (F) Tb volume fraction (BV/TV), (G) Tb thickness, and (H) Tb number. qCT of the mid-femoral shaft was used to determine (I) periosteal circumference and (J) cortical thickness. Data represent the mean ± SEM of measurements taken from at least seven male mice. *P < 0.05; **P < 0.01; ***P < 0.001 compared with vehicle-treated WT mice. †P < 0.05; ††P < 0.01; †††P < 0.001 compared with vehicle-treated β-arrestin2−/− mice; significance was determined with one-way ANOVA with Bonferroni multiple comparisons post test.
Fig. 4
Bone histomorphometry and dynamic indices of bone formation in WT and β-arrestin2−/− mice. (A) Representative nondecalcified, 5-μm sections of lumbar vertebrae isolated from male WT and β-arrestin2−/− mice treated at 9 weeks of age for 8 weeks with vehicle, PTH(1–34), or PTH-βarr. Scale bar, 100 μm. ob, osteoblasts; oc, osteoclasts; os, osteoid. Quantitated histomorphometric analysis of (B) OBS, (C) OS, and (D) OCS after treatment with either vehicle, PTH(1–34), or PTH-βarr. Data represent the mean ± SEM of measurements from four mice. (E) Representative calcein double-labeled, nondecalcified, 10-μm sections of lumbar vertebrae isolated from male WT and β-arrestin2−/− mice treated for 8 weeks with either vehicle, PTH, or PTH-βarr. Scale bar, 100 μm. Bone growth is determined by measuring the distance between calcein-labeled layers (arrows). Quantitation of the (F) mineral apposition rate and (G) bone formation rates from calcein-labeled trabecular bone. Data represent the mean ± SEM of measurements from four mice. *P < 0.05; **P < 0.01; ***P < 0.001 compared with vehicle-treated mice. †P < 0.05; ††P < 0.01; †††P < 0.001 compared with vehicle-treated β-arrestin2−/− mice; significance determined with one-way ANOVA with Bonferroni correction.
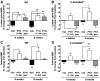
Fig. 5
PTH-βarr increases serum osteocalcin but has no effect on urine DPD excretion. Osteocalcin was measured in serum obtained from male (A) WT and (B) β-arrestin2−/− mice after 4 and 8 weeks of treatment with vehicle, PTH(1–34), or PTH-βarr. Twenty-four-hour urine DPD was measured in male (C) WT and (D) β-arrestin2−/− mice after 4 and 8 weeks of treatment with vehicle, PTH(1–34), or PTH-βarr. Mice were treated starting at 9 weeks of age. Data represent the mean ± SEM of measurements taken from at least seven male mice. *P < 0.05; **P < 0.01; ***P < 0.001 compared with vehicle-treated WT mice. †P < 0.05; ††P < 0.01; †††P < 0.001 compared with vehicle-treated β-arrestin2−/− mice; significance was determined with one-way ANOVA with Bonferroni multiple comparisons post test.
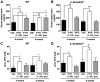
Fig. 6
PTH-βarr induces β-arrestin–dependent expression of osteocalcin but not RANKL or OPG. mRNA was isolated from the calvaria of male WT and β-arrestin2−/− mice treated with vehicle, PTH(1–34), or PTH-βarr, and quantitative RT-PCR was used to determine relative gene expression of protein modulators of bone metabolism: (A) osteocalcin, (B) RANKL, and (C) OPG. Data represent the mean ± SEM from at least six mice. *P < 0.05; **P < 0.01; ***P < 0.001 compared with vehicle-treated WT mice. †P < 0.05; †††P < 0.001 compared with vehicle-treated β-arrestin2−/− mice; significance was determined with one-way ANOVA with Bonferroni multiple comparisons post test.
Similar articles
- β-arrestin-biased agonism at the parathyroid hormone receptor uncouples bone formation from bone resorption.
Bohinc BN, Gesty-Palmer D. Bohinc BN, et al. Endocr Metab Immune Disord Drug Targets. 2011 Jun;11(2):112-9. doi: 10.2174/187153011795564151. Endocr Metab Immune Disord Drug Targets. 2011. PMID: 21476967 Review. - Biased agonism at the parathyroid hormone receptor: a demonstration of functional selectivity in bone metabolism.
Bohinc BN, Gesty-Palmer D. Bohinc BN, et al. Mini Rev Med Chem. 2012 Aug;12(9):856-65. doi: 10.2174/138955712800959125. Mini Rev Med Chem. 2012. PMID: 22681253 Review. - Arrestins in bone.
Bohinc BN, Gesty-Palmer D. Bohinc BN, et al. Prog Mol Biol Transl Sci. 2013;118:335-58. doi: 10.1016/B978-0-12-394440-5.00013-9. Prog Mol Biol Transl Sci. 2013. PMID: 23764060 Review. - Beta-arrestin-biased parathyroid hormone ligands: a new approach to the development of agents that stimulate bone formation.
Ferrari SL, Bouxsein ML. Ferrari SL, et al. Sci Transl Med. 2009 Oct 7;1(1):1ps1. doi: 10.1126/scitranslmed.3000268. Sci Transl Med. 2009. PMID: 20368152 - β-arrestin-selective G protein-coupled receptor agonists engender unique biological efficacy in vivo.
Gesty-Palmer D, Yuan L, Martin B, Wood WH 3rd, Lee MH, Janech MG, Tsoi LC, Zheng WJ, Luttrell LM, Maudsley S. Gesty-Palmer D, et al. Mol Endocrinol. 2013 Feb;27(2):296-314. doi: 10.1210/me.2012-1091. Epub 2013 Jan 11. Mol Endocrinol. 2013. PMID: 23315939 Free PMC article.
Cited by
- Biased Agonism at Nociceptin/Orphanin FQ Receptors: A Structure Activity Study on N/OFQ(1-13)-NH2.
Pacifico S, Ferrari F, Albanese V, Marzola E, Neto JA, Ruzza C, Calò G, Preti D, Guerrini R. Pacifico S, et al. J Med Chem. 2020 Oct 8;63(19):10782-10795. doi: 10.1021/acs.jmedchem.9b02057. Epub 2020 Sep 24. J Med Chem. 2020. PMID: 32901477 Free PMC article. - Phenotypic regulation of the sphingosine 1-phosphate receptor miles apart by G protein-coupled receptor kinase 2.
Burczyk M, Burkhalter MD, Blätte T, Matysik S, Caron MG, Barak LS, Philipp M. Burczyk M, et al. Biochemistry. 2015 Jan 27;54(3):765-75. doi: 10.1021/bi501061h. Epub 2015 Jan 15. Biochemistry. 2015. PMID: 25555130 Free PMC article. - Minireview: More than just a hammer: ligand "bias" and pharmaceutical discovery.
Luttrell LM. Luttrell LM. Mol Endocrinol. 2014 Mar;28(3):281-94. doi: 10.1210/me.2013-1314. Epub 2014 Jan 16. Mol Endocrinol. 2014. PMID: 24433041 Free PMC article. Review. - Multiple ligand-specific conformations of the β2-adrenergic receptor.
Kahsai AW, Xiao K, Rajagopal S, Ahn S, Shukla AK, Sun J, Oas TG, Lefkowitz RJ. Kahsai AW, et al. Nat Chem Biol. 2011 Aug 21;7(10):692-700. doi: 10.1038/nchembio.634. Nat Chem Biol. 2011. PMID: 21857662 Free PMC article. - Local delivery of a novel PTHrP via mesoporous bioactive glass scaffolds to improve bone regeneration in a rat posterolateral spinal fusion model.
Liang B, Huang J, Xu J, Li X, Li J. Liang B, et al. RSC Adv. 2018 Apr 3;8(22):12484-12493. doi: 10.1039/c8ra00870a. eCollection 2018 Mar 26. RSC Adv. 2018. PMID: 35539368 Free PMC article.
References
- Qin L, Raggatt LJ, Partridge NC. Parathyroid hormone: A double-edged sword for bone metabolism. Trends Endocrinol Metab. 2004;15:60–65. - PubMed
- Hock JM, Gera I. Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J Bone Miner Res. 1992;7:65–72. - PubMed
- Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology. 1995;136:3632–3638. - PubMed
- Girotra M, Rubin MR, Bilezikian JP. The use of parathyroid hormone in the treatment of osteoporosis. Rev Endocr Metab Disord. 2006;7:113–121. - PubMed
- Koh AJ, Beecher CA, Rosol TJ, McCauley LK. 3′,5′-Cyclic adenosine monophosphate activation in osteoblastic cells: Effects on parathyroid hormone-1 receptors and osteoblastic differentiation in vitro. Endocrinology. 1999;140:3154–3162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK055524/DK/NIDDK NIH HHS/United States
- HL16037/HL/NHLBI NIH HHS/United States
- R01 HL016037/HL/NHLBI NIH HHS/United States
- DK64353/DK/NIDDK NIH HHS/United States
- R01 HL070631/HL/NHLBI NIH HHS/United States
- HL70631/HL/NHLBI NIH HHS/United States
- HD043446/HD/NICHD NIH HHS/United States
- K12 HD043446/HD/NICHD NIH HHS/United States
- R01 DK064353/DK/NIDDK NIH HHS/United States
- DK55524/DK/NIDDK NIH HHS/United States
- R56 DK055524/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- T32 DK007012/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials