Cutting edge: human latency-associated peptide+ T cells: a novel regulatory T cell subset - PubMed (original) (raw)
Cutting edge: human latency-associated peptide+ T cells: a novel regulatory T cell subset
Roopali Gandhi et al. J Immunol. 2010.
Abstract
Regulatory T cells (Tregs) play an important role in the maintenance of peripheral tolerance. Several molecules including TGF-beta have been linked to the function and differentiation of Tregs. In this study, we describe a unique population of T cells expressing a membrane bound form of TGF-beta, the latency-associated peptide (LAP), and having regulatory properties in human peripheral blood. These CD4(+)LAP(+) T cells lack Foxp3 but express TGF-betaR type II and the activation marker CD69. CD4(+)LAP(+) T cells are hypoproliferative compared with CD4(+)LAP(-) T cells, secrete IL-8, IL-9, IL-10, IFN-gamma, and TGF-beta upon activation, and exhibit TGF-beta- and IL-10-dependent suppressive activity in vitro. The in vitro activation of CD4(+)LAP(-) T cells results in the generation of LAP(+) Tregs, which is further amplified by IL-8. In conclusion, we have characterized a novel population of human LAP(+) Tregs that is different from classic CD4(+)Foxp3(+)CD25(high) natural Tregs.
Conflict of interest statement
Disclosures: The authors have no financial conflicts of interest.
Figures
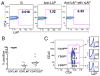
FIGURE 1
Identification of LAP+CD4+ T cells in human peripheral blood. Human PBMCs were isolated and analyzed by FACS. A, Left panel shows annexin−AAD−CD3+CD4+ T cells stained with anti-LAP Ab. Right panels show T cells stained with anti-LAP Ab in presence of rLAP. B, Percentage of CD4+LAP+, CD4+LAP−, and CD4+CD25high T cells among different individuals. C, Left panel shows gating criteria based on CD25 expression into CD25high, CD25int, and CD25low subpopulations. Right panels indicate overlay histogram plots representing the LAP+ T cell subpopulation in respective CD25high, CD25int, and CD25low T cell subpopulations. The isotype control is the filled profile, and anti-LAP staining is the empty profile. The percentages of LAP+ T cells are indicated in each plot. Results are representative of five independent experiments.
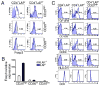
FIGURE 2
Selective expression of activation and regulatory markers on CD4+LAP+ T cells. A, Foxp3 staining of CD25high, CD25int, CD25low LAP+, and LAP- T cells. Cells were stained with AAD, annexin, CD3, CD4, CD25, and LAP followed by intracellular staining for Foxp3. B, RT-PCR analysis to determine Foxp3 expression in respective FACS-sorted populations. C, Human PBMCs were stained with AAD, annexin, CD3, CD4, CD25, LAP, and specific markers including TGF-βRII, CD69, CD103, HLA-DR, and CD3. Filled blue profiles represent isotype control; empty profiles represent specific staining for different Abs on selected populations. The percentage of positive cells are indicated in each plot. Results are representative of five independent experiments.
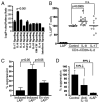
FIGURE 3
Proliferation and in vitro regulatory activity of CD4+LAP+ T cells. A, CD4+LAP−, CD4+LAP+, and CD4+LAP−CD25high T cells were sorted and stimulated with anti-CD3 and anti-CD28 for 5 d. Proliferation of respective T cell subpopulations is represented as a mean (±SD) of three independent experiments. B, Responder T cells were cocultured with Treg subpopulations in presence of anti-CD3− and anti-CD28−coated beads. Percent suppression for each population is represented as mean (±SD) of three independent experiments. C, Suppressive activity of CD4+LAP+ T cells and CD4+LAP−CD25high T cells reversed by blocking TGF-β (rLAP, 25 μg/ml) and anti–IL-10 (20 μg/ml). Results are representative of three independent experiments.
FIGURE 4
Signaling events in CD4+LAP+ T cells and activation-induced generation of CD4+LAP+ T cells. A, CD4+LAP+ and CD4+LAP− T cells were analyzed by reverse-phase protein array to identify specific cytokine signaling pathways active in CD4+LAP+ T cells. B, CD4+LAP− T cells were cultured in presence of anti-CD3, anti-CD28, and IL-2 in addition to either no cytokine (control) or IL-8 or IL-17 for 6 d. Percentage of CD4+LAP+ T cells post-activation was calculated, assigning the control condition as 100%. C, Activation-induced CD4+LAP+ T cells are more suppressive compared with activation-induced CD4+LAP− T cell population and ex vivo-isolated CD4+LAP+ T cells. Percent suppression for each population is represented as mean (±SD) of three independent experiments. D, Activation-induced CD4+LAP+ T cell suppression is reversed by anti–IL-10 and rLAP. In C and D, a representative experiment of three independent experiments is shown.
Similar articles
- CD69+ CD4+ CD25- T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1.
Han Y, Guo Q, Zhang M, Chen Z, Cao X. Han Y, et al. J Immunol. 2009 Jan 1;182(1):111-20. doi: 10.4049/jimmunol.182.1.111. J Immunol. 2009. PMID: 19109141 - Latency-associated peptide identifies a novel CD4+CD25+ regulatory T cell subset with TGFbeta-mediated function and enhanced suppression of experimental autoimmune encephalomyelitis.
Chen ML, Yan BS, Bando Y, Kuchroo VK, Weiner HL. Chen ML, et al. J Immunol. 2008 Jun 1;180(11):7327-37. doi: 10.4049/jimmunol.180.11.7327. J Immunol. 2008. PMID: 18490732 Free PMC article. - Expression of IL-37 contributes to the immunosuppressive property of human CD4+CD25+ regulatory T cells.
Shuai X, Wei-min L, Tong YL, Dong N, Sheng ZY, Yao YM. Shuai X, et al. Sci Rep. 2015 Sep 28;5:14478. doi: 10.1038/srep14478. Sci Rep. 2015. PMID: 26411375 Free PMC article. - TH2 cells in the pathogenesis of airway remodeling: regulatory T cells a plausible panacea for asthma.
McGee HS, Agrawal DK. McGee HS, et al. Immunol Res. 2006;35(3):219-32. doi: 10.1385/IR:35:3:219. Immunol Res. 2006. PMID: 17172648 Review. - Role of endogenous and induced regulatory T cells during infections.
Wohlfert E, Belkaid Y. Wohlfert E, et al. J Clin Immunol. 2008 Nov;28(6):707-15. doi: 10.1007/s10875-008-9248-6. Epub 2008 Sep 23. J Clin Immunol. 2008. PMID: 18810611 Free PMC article. Review.
Cited by
- Downregulation of CD4+LAP+ and CD4+CD25+ regulatory T cells in acute coronary syndromes.
Lin YZ, Lu SH, Lu ZD, Huang Y, Shi Y, Liu L, Wang XY, Ji QW. Lin YZ, et al. Mediators Inflamm. 2013;2013:764082. doi: 10.1155/2013/764082. Epub 2013 Dec 10. Mediators Inflamm. 2013. PMID: 24385687 Free PMC article. - Sec13 Regulates Expression of Specific Immune Factors Involved in Inflammation In Vivo.
Moreira TG, Zhang L, Shaulov L, Harel A, Kuss SK, Williams J, Shelton J, Somatilaka B, Seemann J, Yang J, Sakthivel R, Nussenzveig DR, Faria AM, Fontoura BM. Moreira TG, et al. Sci Rep. 2015 Dec 3;5:17655. doi: 10.1038/srep17655. Sci Rep. 2015. PMID: 26631972 Free PMC article. - Association of gestational diabetes mellitus and negative modulation of the specific humoral and cellular immune response against Toxoplasma gondii.
Oliveira-Scussel ACM, Ferreira PTM, Resende RS, Ratkevicius-Andrade CM, Gomes AO, Paschoini MC, De Vito FB, Farnesi-de-Assunção TS, da Silva MV, Mineo JR, Rodrigues DBR, Rodrigues V. Oliveira-Scussel ACM, et al. Front Immunol. 2022 Sep 20;13:925762. doi: 10.3389/fimmu.2022.925762. eCollection 2022. Front Immunol. 2022. PMID: 36203592 Free PMC article. - Phenotypic and functional characteristic of a newly identified CD8+ Foxp3- CD103+ regulatory T cells.
Liu Y, Lan Q, Lu L, Chen M, Xia Z, Ma J, Wang J, Fan H, Shen Y, Ryffel B, Brand D, Quismorio F, Liu Z, Horwitz DA, Xu A, Zheng SG. Liu Y, et al. J Mol Cell Biol. 2014 Feb;6(1):81-92. doi: 10.1093/jmcb/mjt026. Epub 2013 Jul 15. J Mol Cell Biol. 2014. PMID: 23861553 Free PMC article.
References
- Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. - PubMed
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. - PubMed
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. - PubMed
- Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials