Direct visualization of large and protein-free hemifusion diaphragms - PubMed (original) (raw)
Direct visualization of large and protein-free hemifusion diaphragms
Jörg Nikolaus et al. Biophys J. 2010.
Abstract
Fusion of cellular membranes is a ubiquitous biological process requiring remodeling of two phospholipid bilayers. We believe it is very likely that merging of membranes proceeds via similar sequential intermediates. Contacting membranes form a stalk between the proximal leaflets that expands radially into an hemifusion diaphragm (HD) and subsequently open to a fusion pore. Although considered to be a key intermediate in fusion, direct experimental verification of this structure is difficult due to its transient nature. Using confocal fluorescence microscopy we have investigated the fusion of giant unilamellar vesicles (GUVs) containing phosphatidylserine and fluorescent virus derived transmembrane peptides or membrane proteins in the presence of divalent cations. Time-resolved imaging revealed that fusion was preceded by displacement of peptides and fluorescent lipid analogs from the GUV-GUV adhesion region. A detailed analysis of this area being several mum in size revealed that peptides were completely sequestered as expected for an HD. Lateral distribution of lipid analogs was consistent with formation of an HD but not with the presence of two adherent bilayers. Formation and size of the HD were dependent on lipid composition and peptide concentration.
Copyright (c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
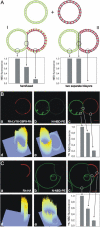
Sequence of fusion between GUVs made of DOPC/DOPE/DOPS (3:1:1, mol/mol/mol), containing either 1 mol % Rh-LLV16-Rh (indicated by an arrow) or 1 mol % N-NBD-PE. Pairs of GUVs were imaged by fluorescence microscopy (rhodamine fluorescence) at 25°C. On addition of 6 mM Ca2+ fusion was monitored. The first image corresponding to t = 0 refers to the last snapshot before alterations of the adhesion region between two GUVs were observed. Magnifications of selected images are shown. Arrows indicate the dimension of the developing HD. Bright spot in the lower figure part corresponds to fluorescent aggregates inside the large GUV. In the last image the GUVs disintegrate. Scale bar = 5 _μ_m.
Figure 2
Fluorescence intensity of fluorescent lipid analogs in the contact region. (A) Expected fluorescence intensity of N-NBD-PE in membranes of adherent GUVs (left GUV labeled with N-NBD-PE (green); right GUV with inserted peptide (red)). Intensity is shown for two possible different structures of the adhesion region: (I) HD; (II) two separate adherent bilayers. Although no N-NBD-PE is found in the peptide-containing GUV for II, the outer leaflet of the peptide-containing GUV becomes labeled by the lipid analog for I. However, NBD intensity is reduced by ∼50% due to FRET from NBD to Rh-labeled peptides. (B and C) GUVs containing the peptide Rh-LV16-G8P9-Rh (B) or Rh-HA (C) and N-NBD-PE labeled GUVs were mixed. A total of 2 mM Ca2+ or Mg2+ were added to trigger adhesion of GUVs. Distribution of (a) Rh-labeled peptide; (b) distribution of N-NBD-PE; (c) overlay of a and b. Fluorescence intensity profiles of (d) rhodamine and (e) NBD. N-NBD-PE fluorescence intensity in three different bilayer regions is given in (f). Region of the NBD-labeled GUV outside the HD (intensity was set to 100%), HD, and region of the peptide-containing GUV outside the HD. Differences between B and C with respect to the relative intensities are due to the different sizes of GUVs.
Figure 3
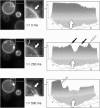
Temporary enrichment of TMDs at the rim of the forming HD. CCD camera images of the fusion kinetic of Fig. 1 are presented in an intensity plot showing the forming HD and its rim. On formation of the HD (see fluorescence decrease in the forming HD (large open arrow)) there is a temporary local fluorescence increase at the rim of the forming HD (small solid arrows) as the TMD gets sequestered. The small open arrow marks structures in the GUV not related to fusion. Note the large open arrow in the intensity plots indicates also the direction of view (from back to front).
Figure 4
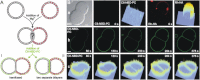
Lipids in the outer leaflet cannot enter the HD. C6-NBD-PC was added to pairs of GUVs with sequestered Rh-HA peptides. After insertion of the lipid analog in the outer leaflet, labeling of the contact region was studied by following the lateral distribution of the NBD fluorescence. (A) Sketch of C6-NBD-PC localization. In case of HD formation no redistribution of the lipid analog to the HD is observed (I) whereas the adhesion region becomes labeled when it is formed by two separated bilayers (II). (B) Lateral distribution of C6-NBD-PC observed by confocal fluorescence microscopy. (a) Images of a GUV pair before addition of C6-NBD-PC (t = 0). From left to right: Differential interference contrast; distribution of C6-NBD-PC (green); intensity profile of NBD fluorescence; distribution of Rh-labeled peptide (red); intensity profile of rhodamine fluorescence; (b) distribution of C6-NBD-PC and (c) corresponding intensity profile at various times after addition of C6-NBD-PC. Scale bar = 5 _μ_m.
Figure 5
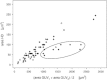
HD area versus GUV surface area. HD area plotted against the mean surface area of the two hemifused GUVs. (S_olid symbols_) GUVs containing 20 mol % PS lipids. (Open symbols) GUVs with 10 mol % PS. A shallower dependence was observed in case the size of the two hemifused GUVs was very different (encircled, ratio of GUV diameters >4).
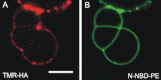
Figure 6
Sequestering of full length HA from contact regions. HA was labeled with TMR and reconstituted into GUVs made of DOPC/DOPE/DOPS (3:1:1, mol/mol/mol), containing 1 mol % N-NBD-PE. On addition of 2 mM Ca2+ adhesion of GUVs and formation of regions depleted of HA could be observed. (A) TMR-HA and (B) N-NBD-PE fluorescence. See the Supporting Material for details. Scale bar = 5 _μ_m.
Similar articles
- Adhesion and merging of lipid bilayers: a method for measuring the free energy of adhesion and hemifusion.
Sun Y, Lee CC, Huang HW. Sun Y, et al. Biophys J. 2011 Feb 16;100(4):987-95. doi: 10.1016/j.bpj.2011.01.013. Biophys J. 2011. PMID: 21320443 Free PMC article. - Confocal microscopic observation of fusion between baculovirus budded virus envelopes and single giant unilamellar vesicles.
Kamiya K, Kobayashi J, Yoshimura T, Tsumoto K. Kamiya K, et al. Biochim Biophys Acta. 2010 Sep;1798(9):1625-31. doi: 10.1016/j.bbamem.2010.05.011. Epub 2010 May 19. Biochim Biophys Acta. 2010. PMID: 20493165 - The use of hemifusion to create asymmetric giant unilamellar vesicles: Insights on induced order domains.
Enoki TA. Enoki TA. Methods Enzymol. 2024;700:127-159. doi: 10.1016/bs.mie.2024.03.025. Epub 2024 Apr 6. Methods Enzymol. 2024. PMID: 38971598 - Elementary Processes and Mechanisms of Interactions of Antimicrobial Peptides with Membranes-Single Giant Unilamellar Vesicle Studies.
Hasan M, Yamazaki M. Hasan M, et al. Adv Exp Med Biol. 2019;1117:17-32. doi: 10.1007/978-981-13-3588-4_3. Adv Exp Med Biol. 2019. PMID: 30980351 Review. - Quantitative optical microscopy and micromanipulation studies on the lipid bilayer membranes of giant unilamellar vesicles.
Bagatolli LA, Needham D. Bagatolli LA, et al. Chem Phys Lipids. 2014 Jul;181:99-120. doi: 10.1016/j.chemphyslip.2014.02.009. Epub 2014 Mar 13. Chem Phys Lipids. 2014. PMID: 24632023 Review.
Cited by
- A supramolecular system mimicking the infection process of an enveloped virus through membrane fusion.
Furukawa H, Kimura Y, Inaba H, Matsuura K. Furukawa H, et al. Sci Rep. 2023 Nov 15;13(1):19934. doi: 10.1038/s41598-023-47347-7. Sci Rep. 2023. PMID: 37968508 Free PMC article. - Sculpting and fusing biomimetic vesicle networks using optical tweezers.
Bolognesi G, Friddin MS, Salehi-Reyhani A, Barlow NE, Brooks NJ, Ces O, Elani Y. Bolognesi G, et al. Nat Commun. 2018 May 14;9(1):1882. doi: 10.1038/s41467-018-04282-w. Nat Commun. 2018. PMID: 29760422 Free PMC article. - Quantification of Giant Unilamellar Vesicle Fusion Products by High-Throughput Image Analysis.
Caliari A, Hanczyc MM, Imai M, Xu J, Yomo T. Caliari A, et al. Int J Mol Sci. 2023 May 4;24(9):8241. doi: 10.3390/ijms24098241. Int J Mol Sci. 2023. PMID: 37175944 Free PMC article. - SNARE-mediated membrane fusion is a two-stage process driven by entropic forces.
McDargh ZA, Polley A, O'Shaughnessy B. McDargh ZA, et al. FEBS Lett. 2018 Nov;592(21):3504-3515. doi: 10.1002/1873-3468.13277. Epub 2018 Nov 2. FEBS Lett. 2018. PMID: 30346036 Free PMC article. - Highly Efficient Protein-free Membrane Fusion: A Giant Vesicle Study.
Lira RB, Robinson T, Dimova R, Riske KA. Lira RB, et al. Biophys J. 2019 Jan 8;116(1):79-91. doi: 10.1016/j.bpj.2018.11.3128. Epub 2018 Dec 1. Biophys J. 2019. PMID: 30579564 Free PMC article.
References
- Kozlov M.M., Markin V.S. Possible mechanism of membrane fusion. Biofizika. 1983;28:242–247. - PubMed
- Chernomordik L.V., Melikyan G.B., Chizmadzhev Y.A. Biomembrane fusion: a new concept derived from model studies using two interacting planar lipid bilayers. Biochim. Biophys. Acta. 1987;906:309–352. - PubMed
- Chernomordik L.V., Kozlov M.M. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 2003;72:175–207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources