CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer's disease-like pathogenesis in APP+PS1 bigenic mice - PubMed (original) (raw)
CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer's disease-like pathogenesis in APP+PS1 bigenic mice
Tomomi Kiyota et al. FASEB J. 2010 Aug.
Abstract
Cytokines play an emerging role as neurotransmitters, neuromodulators, and neurohormones in the brain. This paradigm shift in cytokine function offers a new framework to understand their roles in ameliorating neurodegenerative disorders, such as Alzheimer's disease (AD). Molecular adjuvant therapy of AD animal models with glatiramer acetate induces anti-inflammatory responses and therapeutic effects. Although these effects are potentially mediated through anti-inflammatory cytokine signaling, the exact molecular identities and pathways are poorly understood. Here, we show that virus-mediated expression of the mouse interleukin (IL)-4 gene in beta-amyloid precursor protein + presenilin-1 (APP+PS1) bigenic mice attenuates AD pathogenesis. Introduction of an adeno-associated viral (AAV) vector encoding IL-4 into the hippocampus resulted in sustained expression of IL-4, reduced astro/microgliosis, amyloid-beta peptide (Abeta) oligomerization and deposition, and enhanced neurogenesis. Moreover, increased levels of IL-4 improved spatial learning, promoted phosphorylation of N-methyl-D-aspartate receptor subunit 2B at Tyr 1472, and enhanced its cell surface retention both in vivo and in vitro. Our data suggest that neuronal anti-inflammatory cytokine signaling may be a potential alternative target for non-Abeta-mediated treatment of AD.
Figures
Figure 1
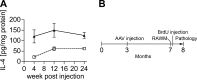
AAV injection and strategy. A) AAV-mediated somatic gene transfer of IL-4. Non-Tg mice at 3 mo of age received bilateral hippocampal injection of AAV-GFP (2×109 viral particles (VP)/hippocampus, dotted line) or AAV-IL-4 (2×109 VP/hippocampus, solid line), were sacrificed 4, 12, and 24 wk postinjection. The amounts of IL-4 in mouse hippocampal protein extracts were quantified by enzyme-linked immunosorbent assay, showing sustained expression of IL-4. Error bars represent
se
(_n_=3). B) Experimental design. APP+PS1 mice received bilateral hippocampal injection of AAV-GFP or AAV-IL-4 at 3 mo of age and were tested by 2-d RAWM task at 7 mo of age. Non-Tg mice served as a positive control for the spatial learning task. Mice were intraperitoneally injected with BrdU 5 times for 2.5 d, 21 d before being sacrificed at 8 mo of age.
Figure 2
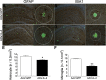
Gene delivery of IL-4 suppresses glial accumulation in APP+PS1 mice. A_–_D ) APP+PS1 mice injected with AAV-GFP (A, B) or AAV-IL-4 (C, D) at 3 mo of age were sacrificed at 8 mo of age. Hippocampal frozen sections were immunostained for GFAP (astrocyte; A, C) or IBA1 (microglia; B, D), and counterstained by TS. Scale bars = 200 μm (low magnification; left); 40 μm (high magnification; right). E, F) Quantification of GFAP+ (E) or IBA1+ (F) cells found within the circle surrounding TS+ Aβ plaques. Radii of outer concentric circles in GFAP+ cells were 100 μm greater than the inner circles that surrounded the compact plaques (A, C), and 50 μm greater in IBA1+ cells (B, D). Error bars =
se
(_n_=5/group, 10 sections/brain). *P < 0.05, **P < 0.01 vs. AAV-GFP group; Student’s t test.
Figure 3
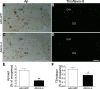
Aβ deposition in the hippocampal region of gene-delivered APP+PS1 mouse brain. A_–_D) Frozen brain sections of APP+PS1 mice injected with AAV-GFP (A, B) or AAV-IL-4 (C, D) were immunostained with anti-Aβ antibody (A, C) and counterstained by TS for compact plaque (B, D). E, F) Total Aβ load (E) and TS+ area (F) in hippocampal regions were quantified in APP+PS1 mice injected with AAV-GFP (GFP) or AAV-IL-4 (IL-4). Error bars =
se
(_n_=5/group, 10 sections/brain). *P < 0.05, **P < 0.01 vs. AAV-GFP group; Student’s t test. Scale bar = 500 μm.
Figure 4
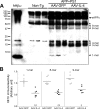
Aβ oligomer formation in the hippocampus of gene-delivered APP+PS1 mouse brain. A) Immunoblotting using 6E10 antibody showed specific Aβ oligomers in the extracellular_-_enriched fraction of AAV-injected APP+PS1 mouse hippocampus. Arrowheads indicate respective migration positions of multimers. Arrow indicates sAPPα fragments processed from full-length APP. Asterisk indicates nonspecific band. B) Band luminescent intensities for 1-, 8-, and 9-mers were quantified by Image J software. Bars represent average values (_n_=6/group). *P < 0.05, **P < 0.01 vs. AAV-GFP group; Student’s t test.
Figure 5
Gene delivery of IL-4 enhances neurogenesis in SGZ of APP+PS1 mice. A_–_C) Representative images of Dcx staining in the dentate gyrus of non-Tg (A) or APP+PS1 mice injected with AAV-GFP (B) or AAV-IL-4 (C) into the hippocampus at 3 mo of age, and intraperitoneally injected with BrdU 3 wk prior to euthanasia at 8 mo of age. D_–_F) Immunofluorescence of BrdU (green) and NeuN (red) in the dentate gyrus of non-Tg (D) or APP+PS1 mice injected with AAV-GFP (E) or AAV-IL-4 (F). G, H) Quantification of Dcx+ (G) or BrdU+Neu+ cells (H) in SGZ. Error bars =
se
(_n_=5/group, 10 sections/brain). *P < 0.05, ***P < 0.001 vs. non-Tg group; #P < 0.05, ##P < 0.01 vs. AAV-GFP group; 1-way ANOVA and Newman-Keuls posttest. Scale bars = 200 μm (C); 100 μm (F).
Figure 6
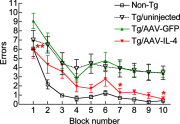
Gene delivery of IL-4 improves memory function of APP+PS1 mice. APP+PS1 mice received bilateral hippocampal injection of AAV-GFP or AAV-IL-4 at 3 mo of age and were tested by the 2-d RAWM task at 7–8 mo of age. Non-Tg and uninjected APP+PS1 mice (Tg/uninjected group) serve as positive/negative controls for the spatial learning task. Error bars =
se
(_n_=8/group). *P < 0.05, **P < 0.01 vs. AAV-GFP group; 2-way repeated-measures ANOVA and Bonferroni post hoc test.
Figure 7
IL-4 promotes the expression and phosphorylation of NR2B. A) Immunoblotting (top) and quantification (bottom) of NR2A and NR2B in the membrane-enriched fraction of the mouse hippocampus in non-Tg (NTg), uninjected APP+PS1 (uninj.), APP+PS1 mice injected with AAV-GFP (GFP), and APP+PS1 mice injected with AAV-IL-4 (IL-4). B) Immunoblotting (top) and quantification (bottom) of phospho-Tyr1472 NR2B (pNR2B) in the same fraction of the hippocampus. *P < 0.05, **P < 0.01, ***P < 0.001 vs. NTg group; #P < 0.05, ###P < 0.001 vs. AAV-GFP group; 1-way ANOVA and Newman-Keuls post hoc test. Error bars =
se
(_n_=6/group). C) Immunoblotting (top) and quantification (bottom) of pNR2B, NR2B, and β-actin in IL-4-treated primary cultured neurons. D) Immunoblotting (top) and quantification (bottom) of biotinylated NR2B at the cell surface level, total NR2B in IL-4 and/or oligomeric Aβ42-treated primary cultured neurons. **P < 0.01, ***P < 0.001 vs. control; ##P < 0.01 vs. Aβ42 group; 1-way ANOVA and Newman-Keuls post hoc test. Error bars represent
se
(_n_=3/group). Con, control; N.D., no significant difference.
Similar articles
- AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice.
Kiyota T, Ingraham KL, Swan RJ, Jacobsen MT, Andrews SJ, Ikezu T. Kiyota T, et al. Gene Ther. 2012 Jul;19(7):724-33. doi: 10.1038/gt.2011.126. Epub 2011 Sep 15. Gene Ther. 2012. PMID: 21918553 Free PMC article. - Urolithin A attenuates memory impairment and neuroinflammation in APP/PS1 mice.
Gong Z, Huang J, Xu B, Ou Z, Zhang L, Lin X, Ye X, Kong X, Long D, Sun X, He X, Xu L, Li Q, Xuan A. Gong Z, et al. J Neuroinflammation. 2019 Mar 14;16(1):62. doi: 10.1186/s12974-019-1450-3. J Neuroinflammation. 2019. PMID: 30871577 Free PMC article. - Long-term treadmill exercise inhibits the progression of Alzheimer's disease-like neuropathology in the hippocampus of APP/PS1 transgenic mice.
Liu HL, Zhao G, Zhang H, Shi LD. Liu HL, et al. Behav Brain Res. 2013 Nov 1;256:261-72. doi: 10.1016/j.bbr.2013.08.008. Epub 2013 Aug 19. Behav Brain Res. 2013. PMID: 23968591 - Intramuscular delivery of p75NTR ectodomain by an AAV vector attenuates cognitive deficits and Alzheimer's disease-like pathologies in APP/PS1 transgenic mice.
Wang QH, Wang YR, Zhang T, Jiao SS, Liu YH, Zeng F, Li J, Yao XQ, Zhou HD, Zhou XF, Wang YJ. Wang QH, et al. J Neurochem. 2016 Jul;138(1):163-73. doi: 10.1111/jnc.13616. Epub 2016 Jun 6. J Neurochem. 2016. PMID: 26991827 - Overexpression of TIPE2, a Negative Regulator of Innate and Adaptive Immunity, Attenuates Cognitive Deficits in APP/PS1 Mice.
Miao Y, Wang N, Shao W, Xu Z, Yang Z, Wang L, Ju C, Zhang R, Zhang F. Miao Y, et al. J Neuroimmune Pharmacol. 2019 Sep;14(3):519-529. doi: 10.1007/s11481-019-09861-2. Epub 2019 Jul 8. J Neuroimmune Pharmacol. 2019. PMID: 31286344
Cited by
- Mathematical Modeling of Proliferative Immune Response Initiated by Interactions Between Classical Antigen-Presenting Cells Under Joint Antagonistic IL-2 and IL-4 Signaling.
Atitey K, Anchang B. Atitey K, et al. Front Mol Biosci. 2022 Jan 28;9:777390. doi: 10.3389/fmolb.2022.777390. eCollection 2022. Front Mol Biosci. 2022. PMID: 35155574 Free PMC article. - Glatiramer Acetate Immunomodulation: Evidence of Neuroprotection and Cognitive Preservation.
Kasindi A, Fuchs DT, Koronyo Y, Rentsendorj A, Black KL, Koronyo-Hamaoui M. Kasindi A, et al. Cells. 2022 May 7;11(9):1578. doi: 10.3390/cells11091578. Cells. 2022. PMID: 35563884 Free PMC article. Review. - Interleukin-4 affects microglial autophagic flux.
Tang RH, Qi RQ, Liu HY. Tang RH, et al. Neural Regen Res. 2019 Sep;14(9):1594-1602. doi: 10.4103/1673-5374.255975. Neural Regen Res. 2019. PMID: 31089059 Free PMC article. - New Possibilities in the Therapeutic Approach to Alzheimer's Disease.
Doroszkiewicz J, Mroczko B. Doroszkiewicz J, et al. Int J Mol Sci. 2022 Aug 10;23(16):8902. doi: 10.3390/ijms23168902. Int J Mol Sci. 2022. PMID: 36012193 Free PMC article. Review. - Spinal Cord Injury Leads to Hippocampal Glial Alterations and Neural Stem Cell Inactivation.
Jure I, De Nicola AF, Encinas JM, Labombarda F. Jure I, et al. Cell Mol Neurobiol. 2022 Jan;42(1):197-215. doi: 10.1007/s10571-020-00900-8. Epub 2020 Jun 14. Cell Mol Neurobiol. 2022. PMID: 32537668
References
- Selkoe D. J. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6:487–498. - PubMed
- Schenk D., Barbour R., Dunn W., Gordon G., Grajeda H., Guido T., Hu K., Huang J., Johnson-Wood K., Khan K., Kholodenko D., Lee M., Liao Z., Lieberburg I., Motter R., Mutter L., Soriano F., Shopp G., Vasquez N., Vandevert C., Walker S., Wogulis M., Yednock T., Games D., Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. - PubMed
- Morgan D., Diamond D. M., Gottschall P. E., Ugen K. E., Dickey C., Hardy J., Duff K., Jantzen P., DiCarlo G., Wilcock D., Connor K., Hatcher J., Hope C., Gordon M., Arendash G. W. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. - PubMed
- Bard F., Barbour R., Cannon C., Carretto R., Fox M., Games D., Guido T., Hoenow K., Hu K., Johnson-Wood K., Khan K., Kholodenko D., Lee C., Lee M., Motter R., Nguyen M., Reed A., Schenk D., Tang P., Vasquez N., Seubert P., Yednock T. Epitope and isotype specificities of antibodies to beta-amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proc Natl Acad Sci U S A. 2003;100:2023–2028. - PMC - PubMed
- Hock C., Konietzko U., Streffer J. R., Tracy J., Signorell A., Muller-Tillmanns B., Lemke U., Henke K., Moritz E., Garcia E., Wollmer M. A., Umbricht D., de Quervain D. J., Hofmann M., Maddalena A., Papassotiropoulos A., Nitsch R. M. Antibodies against beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003;38:547–554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 NS043985/NS/NINDS NIH HHS/United States
- R21 AG032600/AG/NIA NIH HHS/United States
- R01 MH083523-03/MH/NIMH NIH HHS/United States
- R01 MH083523/MH/NIMH NIH HHS/United States
- R01 MH083523-02/MH/NIMH NIH HHS/United States
- R01 MH083523-01/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases