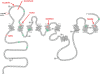Alzheimer's disease phenotypes and genotypes associated with mutations in presenilin 2 - PubMed (original) (raw)
Case Reports
Alzheimer's disease phenotypes and genotypes associated with mutations in presenilin 2
Suman Jayadev et al. Brain. 2010 Apr.
Abstract
Mutations in presenilin 2 are rare causes of early onset familial Alzheimer's disease. Eighteen presenilin 2 mutations have been reported, although not all have been confirmed pathogenic. Much remains to be learned about the range of phenotypes associated with these mutations. We have analysed our unique collection of 146 affected cases in 11 Volga German families, 101 who are likely to have the same N141I mutation in presenilin 2 (54 genotyped confirmed). We have also assessed the detailed neuropathologic findings in 18 autopsies from these families and reviewed the world's literature on other presenilin 2 mutations; presenting a novel mutation that is predicted to lead to a premature truncation codon. Seven presenilin 2 mutations reported in the literature have strong evidence for pathogenicity whereas others may be benign polymorphisms. One hundred and one affected persons, with sufficient historical information from the Volga German pedigrees (N141I mutation), had a mean onset age of 53.7 years+/-7.8 (range 39-75) and mean age at death of 64.2 years+/-9.8 (range 43-88). These figures overlap with and generally fall between the results from the subjects in our centre who have late onset familial Alzheimer's disease or mutations in presenilin 1. Seizures were noted in 20 (30%) of 64 subjects with detailed medical records. Two mutation carriers lived beyond age 80 without developing dementia, representing uncommon examples of decreased penetrance. Two persons had severe amyloid angiopathy and haemorrhagic stroke. Eighteen cases had detailed histopathology available and analysed at our institution. Braak stage was five or six, amyloid angiopathy and neuritic plaques were common and more than 75% had Lewy bodies in the amygdala. TAR DNA-binding protein-43 inclusions were uncommon. In addition, a 58-year-old female with a 2 year course of cognitive decline and no family history of dementia has abnormal fludeoxyglucose-positron emission tomography imaging and a novel 2 base pair deletion in presenilin 2 at nucleotide 342/343, predicted to produce a frame-shift and premature termination. We conclude that mutations in presenilin 2 are rare with only seven being well documented in the literature. The best studied N141I mutation produces an Alzheimer's disease phenotype with a wide range of onset ages overlapping both early and late onset Alzheimer's disease, often associated with seizures, high penetrance and typical Alzheimer's disease neuropathology. A novel premature termination mutation supports loss of function or haploinsufficiency as pathogenic mechanisms in presenilin 2 associated Alzheimer's disease.
Figures
Figure 1
PSEN2 protein showing sites of reported mutations. Probable pathogenic mutations are shown in red. Possible but uncertain mutations are shown in green. The N141I mutation is indicated with an arrow. The K115E fx10 truncation is shown at the red line and its associated mutation is indicated as c.342–343 del GA. Adapted from
http://www.molgen.ua.ac.be/ADMutations
(Cruts and van Broeckhoven, 1998).
Figure 2
R family with the N141I mutation showing high penetrance with 26 known affected persons in five generations. Mean age of onset is 49.5 years and mean age at death is 57.4 years. Individual IV-2 is a phenocopy with dementia but without the PSEN2 mutation. IV-13 and IV-15 had autopsy confirmation of Alzheimer’s disease but sufficient material was not available for our detailed neuropathological study. * = persons that have been genotyped; A = autopsy; black symbols = affected Alzheimer’s disease.
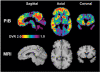
Figure 3
Pittsburgh compound B (PiB) PET and corresponding MRI images from a 52-year-old carrier of a PS2 N141I mutation from the R family with mild Alzheimer’s disease (V-34). The Pittsburgh compound B PET images are parametric distribution volume ratio (DVR) images using 90 min of data and the cerebellum as reference as described in Lopresti et al. (2005). The MRI is a 1.5 T spoiled gradient recalled downsampled to the PET resolution. Pittsburgh compound B uptake is noted prominently in frontal and parietal cortices and caudate.
Similar articles
- Identification of PSEN2 mutation p.N141I in Argentine pedigrees with early-onset familial Alzheimer's disease.
Muchnik C, Olivar N, Dalmasso MC, Azurmendi PJ, Liberczuk C, Morelli L, Brusco LI. Muchnik C, et al. Neurobiol Aging. 2015 Oct;36(10):2674-7.e1. doi: 10.1016/j.neurobiolaging.2015.06.011. Epub 2015 Jun 15. Neurobiol Aging. 2015. PMID: 26166204 - Amyloid angiopathy in a Volga German family with Alzheimer's disease and a presenilin-2 mutation (N141I).
Nochlin D, Bird TD, Nemens EJ, Ball MJ, Sumi SM. Nochlin D, et al. Ann Neurol. 1998 Jan;43(1):131-5. doi: 10.1002/ana.410430124. Ann Neurol. 1998. PMID: 9450781 - Three-year follow-up of a patient with early-onset Alzheimer's disease with presenilin-2 N141I mutation - case report and review of the literature.
Nikisch G, Hertel A, Kiessling B, Wagner T, Krasz D, Hofmann E, Wiedemann G. Nikisch G, et al. Eur J Med Res. 2008 Dec 3;13(12):579-84. Eur J Med Res. 2008. PMID: 19073399 Review. - Identification of PSEN1 mutations p.M233L and p.R352C in Han Chinese families with early-onset familial Alzheimer's disease.
Jiang HY, Li GD, Dai SX, Bi R, Zhang DF, Li ZF, Xu XF, Zhou TC, Yu L, Yao YG. Jiang HY, et al. Neurobiol Aging. 2015 Mar;36(3):1602.e3-6. doi: 10.1016/j.neurobiolaging.2014.11.009. Epub 2014 Dec 18. Neurobiol Aging. 2015. PMID: 25595498 - Gene mutations associated with early onset familial Alzheimer's disease in China: An overview and current status.
Qin Q, Yin Y, Wang Y, Lu Y, Tang Y, Jia J. Qin Q, et al. Mol Genet Genomic Med. 2020 Oct;8(10):e1443. doi: 10.1002/mgg3.1443. Epub 2020 Aug 6. Mol Genet Genomic Med. 2020. PMID: 32767553 Free PMC article. Review.
Cited by
- Alzheimer's disease-associated genotypes differentially influence chronic evoked seizure outcomes and antiseizure medicine activity in aged mice.
Knox KM, Davidson S, Lehmann LM, Skinner E, Lo A, Jayadev S, Barker-Haliski M. Knox KM, et al. bioRxiv [Preprint]. 2024 Oct 7:2024.10.06.616921. doi: 10.1101/2024.10.06.616921. bioRxiv. 2024. PMID: 39416203 Free PMC article. Preprint. - PSEN2 Mutations May Mimic Frontotemporal Dementia: Two New Case Reports and a Review.
Minguillón Pereiro AM, Quintáns Castro B, Ouro Villasante A, Aldrey Vázquez JM, Cortés Hernández J, Aramburu-Núñez M, Arias Gómez M, Jiménez Martín I, Sobrino T, Pías-Peleteiro JM. Minguillón Pereiro AM, et al. Biomedicines. 2024 Aug 17;12(8):1881. doi: 10.3390/biomedicines12081881. Biomedicines. 2024. PMID: 39200345 Free PMC article. - Somatostatin: Linking Cognition and Alzheimer Disease to Therapeutic Targeting.
Sandoval KE, Witt KA. Sandoval KE, et al. Pharmacol Rev. 2024 Oct 16;76(6):1291-1325. doi: 10.1124/pharmrev.124.001117. Pharmacol Rev. 2024. PMID: 39013601 Review. - Mutational Landscape of Alzheimer's Disease and Frontotemporal Dementia: Regional Variances in Northern, Central, and Southern Italy.
Saraceno C, Pagano L, Laganà V, Geviti A, Bagnoli S, Ingannato A, Mazzeo S, Longobardi A, Fostinelli S, Bellini S, Montesanto A, Binetti G, Maletta R, Nacmias B, Ghidoni R. Saraceno C, et al. Int J Mol Sci. 2024 Jun 27;25(13):7035. doi: 10.3390/ijms25137035. Int J Mol Sci. 2024. PMID: 39000146 Free PMC article.
References
- Andreoli V, Trecroci F, La Russa A, Di Palma G, Quattrone A, Cittadella R. Gene symbol: PSEN2. Disease: Alzheimer disease. Hum Genet. 2008;124:304. - PubMed
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–9. - PubMed
- Bernardi L, Tomaino C, Anfossi M, Gallo M, Geracitano S, Puccio G. Late onset familial Alzheimer's disease: novel presenilin 2 mutation and PS1 E318G polymorphism. J Neurol. 2008;255:604–6. - PubMed
- Binetti G, Signorini S, Squitti R, Alberici A, Benussi L, Cassetta E, et al. Atypical dementia associated with a novel presenilin-2 mutation. Ann Neurol. 2003;54:832–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AG025516-02/AG/NIA NIH HHS/United States
- R37 AG025516-06/AG/NIA NIH HHS/United States
- P50 AG005136-24/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- R37 AG025516/AG/NIA NIH HHS/United States
- R37 AG025516-07/AG/NIA NIH HHS/United States
- P01 AG025204-04/AG/NIA NIH HHS/United States
- R37 AG025516-08/AG/NIA NIH HHS/United States
- R37 AG025516-05/AG/NIA NIH HHS/United States
- P01 AG025204/AG/NIA NIH HHS/United States
- P01 AG025204-04S1/AG/NIA NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- R37 AG025516-01/AG/NIA NIH HHS/United States
- R37 AG025516-03/AG/NIA NIH HHS/United States
- P01 AG025204-05/AG/NIA NIH HHS/United States
- P01 AG025204-07/AG/NIA NIH HHS/United States
- R37 AG025516-04/AG/NIA NIH HHS/United States
- RF1 AG025516/AG/NIA NIH HHS/United States
- P01 AG025204-08/AG/NIA NIH HHS/United States
- P01 AG025204-06/AG/NIA NIH HHS/United States
- R37 AG025516-05S1/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical