Altered lipid content inhibits autophagic vesicular fusion - PubMed (original) (raw)
Altered lipid content inhibits autophagic vesicular fusion
Hiroshi Koga et al. FASEB J. 2010 Aug.
Abstract
The autophagic/lysosomal system includes a variety of vesicular compartments that undergo dynamic fusion events. However, the characteristics and factors modulating these interactions remain, for the most part, unknown. To gain insights on the properties that govern lysosomal fusion events, we have established an in vitro fusion assay using different lysosomal/autophagic compartments isolated from mouse liver. We have found that autophagosome/lysosome fusion is a temperature-dependent process (fusion increment of 0.2+/-0.01%/degrees C), which requires ATP (1-3 mM), GTP (1-2 mM), Ca(2+) (0.2-2 mM), and an acidic lysosomal pH (pH 5.2). Furthermore, changes in membrane lipid composition, induced either in vitro, by treatment with 25 mM methyl-beta-cyclodextrin, or in vivo, by subjecting animals to a high-fat-diet challenge (60% kcal in fat) reduce autophagosome/lysosome fusion up to 70% of that observed in untreated fractions or from animals under a normal regular diet. These findings reveal a novel role for lipids in autophagic fusion and provide a mechanism for the reduced macroautophagic rates observed during exposure to a chronic lipid challenge. Changes in the intracellular lipid content (i.e., metabolic disorders) may thus have pronounced effects on the fusion step of macroautophagy and affect the overall activity of this intracellular proteolytic pathway.
Figures
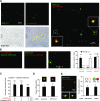
Figure 1
Characterization of labeling of APG and lysosomal fractions. A) APGs and lysosomes (Lys) isolated from mouse liver were subjected to immunoblot for the indicated antibodies. Homo, homogenate; Cyt, cytosol. B) Ultrastructure of the isolated fractions. C) Fluorescence (left panels) and bright field images (right panels) of the same fractions labeled with antibodies against LC3 (APGs) and LAMP-2B (lysosomes), and the corresponding secondary antibodies conjugated with FITC or Cy5, respectively. Percentage of total particles labeled per field is indicated at bottom. D) Analysis of antibody labeling of APGs and lysosomes using FACS analysis. Unlabeled or antibody labeled fractions were subjected to FACS analysis. Particle sorting analysis and percentage of particles detected in each channel are shown for unlabeled APGs (a), LC3-Alexa488 labeled-APGs (b) and unlabeled lysosomes (c), and LAMP-2B-Cy5-labeled- lysosomes (d).
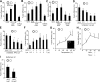
Figure 2
Reconstitution of APG-lysosome fusion. A) Overlapping of bright field and merged fluorescent channels of mouse liver APGs and lysosomes (Lys) labeled as in Fig. 1_C_ and incubated in fusion buffer. Right panel insets: higher-magnification images of fusion events. B) Negligible exchange of antibodies during the fusion reaction. Similarly labeled APG and lysosomes were incubated separately in fusion buffer, and after centrifugation their supernatants (sup) were collected and incubated with labeled lysosomes (left panel) and labeled APGs (left middle panel). Incubation in parallel with the originally labeled fractions is shown as a positive control of fusion (right middle panel). Graph (right panel) shows number of green or red vesicles per field in each of the three reactions. Values are also shown at top of respective bars. C) LAMP-2B-labeled lysosomes were incubated alone or with two different concentrations of unlabeled autophagosomes for 30 min at 37°C. Total number of red particles per field at the end of each of the incubations was quantified. D) Percentage of fusion events between lysosomes labeled with anti-LAMP-2B and APGs either labeled with anti-LC3 or isolated from GFP-LC3 transgenic mice. Values are expressed as percentage of fusion events relative to the total number of particles in the field. E) Fusion events between APGs and lysosomes isolated from cells previously incubated with MDC and with LysoTracker, respectively. Left panel: representative fluorescent image. Insets: examples of fusion events at higher magnification. Right panel: percentage of fusion events between vesicles labeled with antibodies against membrane proteins (as in A) or vesicles with fluorescent content. Values are expressed as in E . All values are means +
se
of 3 different experiments.
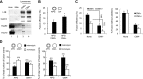
Figure 3
Requirements for heterotypic APG-lysosome fusion. APGs and lysosomes (Lys) isolated from 6-h-starved mouse livers were labeled with antibodies against LC3 (+FITC) and LAMP-2B (+Cy5), respectively. A_–_C) Fractions were diluted to the same final protein concentration (0.01 mg/ml), and fusion efficiency was calculated after incubation at 37°C for 30 min in the presence of different concentrations of ATP and its nonhydrolyzable analog ATPγS (A), GTP and its nonhydrolyzable analog GTPγS (B), or calcium chloride and the calcium chelator EDTA (C). D, E) Fusion efficiency of equal concentrations of lysosomes and APGs incubated with increasing amounts of unlabeled lysosomes (D) or APGs (E). F) APGs and lysosomes were diluted to the same concentration of particles and labeled, and a fixed amount of APGs was incubated with increasing concentrations of lysosomes. G, H) Fusion efficiency of equal concentrations of lysosomes and APGs incubated in fusion medium for 30 min at indicated temperatures (G) or at 37°C for indicated periods of time (H). I) Fusion efficiency of APGs labeled as above and incubated in fusion buffer with untreated lysosomes or lysosomes pretreated with Bafilomycin A1 (B-Lys). Values are expressed as in Fig. 1_D_ and are means +
se
of 3–5 different experiments. *P < 0.05.
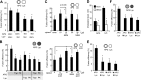
Figure 4
Fusion events between APGs and different lysosomal subpopulations. A) Homogenate (Homo), mitochondria (Mito), and lysosomes with high (CMA+) and low (CMA−) CMA activity isolated from starved mice were subjected to immunoblots for the indicated antibodies. B) APGs CMA+ and CMA− lysosomes isolated from livers of starved mice were labeled with antibodies against LC3, hsc70, and LAMP-2B, respectively, and the corresponding fluorophore-conjugated secondary antibodies. Fractions were incubated for 30 min at 37°C, and fusion events were analyzed. Values are expressed as percentage of fusion events relative to the total number of particles in the field and are means +
se
of 3–5 different experiments. C) APGs and CMA+ and CMA− lysosomes labeled as in B were incubated alone or in the presence of unlabeled CMA− lysosomes, and fusion events were analyzed. Values are expressed as percentage of fusion events relative to the total number of particles in the field (left panel; percentage of decrease in the presence of unlabeled lysosomes is shown) or as percentage of the fusion efficiency in the absence of unlabeled CMA− lysosomes (right panel). Values are means +
se
of 3 different experiments. D) Homotypic and heterotypic fusion events in fusion reactions like those described in B for APGs with CMA+ lysosomes (left panel) or CMA− lysosomes (right panel). Values are expressed as percentage of total fusion reactions and are means +
se
of 3 different experiments. *P < 0.05.
Figure 5
Effect of changes in autophagic activity and in the membrane components of the autophagic-lysosomal fusion. A) APGs and lysosomes (Lys) isolated from livers of mice fed or starved for 6 h before isolation were labeled with anti-LC3 (+FITC) or anti-LAMP-2B (+Cy5), respectively. Values represent percentage of fusion events relative to the total number of particles in the field and are means +
se
of 4 different experiments. B) APGs and Lys isolated from cells previously incubated with MDC and LysoTracker, respectively, were treated with 10 mg/ml of trypsin at room temperature for 15 min or with 10 mg/ml of proteinase K (PK) at 4°C for 15 min. Samples were collected by centrifugation; further action of the proteases was inhibited at the end of the incubation by addition of a cocktail of protease inhibitors. Efficiency of the protease treatment and of the protease inhibitor cocktail was tested by immunoblot as shown in Supplemental Fig. S9. Treated fractions were subjected to in vitro fusion assay. Fusion efficiency is expressed as percentage of fusion events relative to total number of particles in field; values are means +
se
of 3 different experiments. C) Starved mouse APGs labeled as in A were incubated with LAMP-2B-labeled lysosomes (left panel) or Texas-red asialoglycoprotein labeled endosomes (right panel) in fusion buffer alone (none) or supplemented with indicated amounts of cytosol from the same animals. Where indicated, 1 mM ATP, GTP, or NEM was added to the cytosolic fraction. Fusion efficiency was expressed as in A. Values are means +
se
of 3 different experiments. D, E) APGs and Lys isolated from livers of mice starved for 6 h were subjected to treatment with methyl-β-cyclodextrin (M). Levels of cholesterol in treated and untreated fractions were measured (D). Fractions were labeled as in A, and fusion events were analyzed (E). Values are means +
se
of 3 different experiments. F) Fusion events between monodansylcadaverine-labeled APGs and LysoTracker-labeled Lys isolated from NIH-3T3 cells and subjected to the same treatments as in E. Values are means +
se
of 3 different experiments are shown. *P < 0.05.
Figure 6
Lipid stimuli inhibit macroautophagy by reducing APG-lysosome fusion. A) Right panels: degradation of long-lived proteins in hepatocytes cultured in regular medium (RM) or in medium supplemented with 0.25 mM oleate (OL) or palmitate (PAL) without additions (None, left graph) or in the presence of 20 mM ammonium chloride (NH4Cl, right graph). Values are expressed as percentage of proteolysis and are means +
se
of 4 different experiments. Left panel: BODIPY 493/503 staining of the cultured cells to highlight the cellular content of lipid droplets. Nuclei are labeled with DAPI. B) Macroautophagy activity in the same cells was calculated as the percentage of lysosomal degradation (inhibited by NH4Cl) sensitive to 3-methyladenine. Both treatments were added at the beginning of the chase period to avoid any effect of the treatments on protein synthesis. Values are means +
se
of 4 different experiments. C) NIH-3T3 cells were transiently transfected with the GFP-mcherry-LC3 reporter, and the percentage of APGs (yellow) and autophagolysosomes (red) present in untreated cells and cells treated with oleate (0.125, 0.25, and 0.5 mM) or palmitate (0.25, 0.5, and 0.75 mM) for 24 h was determined. Values are means +
se
of 3 different experiments. Representative images are shown; full size fields are shown in Supplemental Fig. S8. D, E) APGs and lysosomes (Lys) isolated from livers of mice fed a regular diet (RD) or an HFD for 16 wk and starved (D) or not (E) for 6 h before isolation were labeled with anti-LC3 (+FITC) or anti-LAMP-2B (+Cy5), respectively. Incubations of same fractions at 4°C did not reveal significant differences in background fusion between these compartments. Values are expressed as percentage of fusion events relative to total number of particles in the field and are means +
se
of 4 different experiments. *,§P < 0.05.
Figure 7
Enhanced interaction of APG with the endocytic compartment during a lipid stimulus. A) APGs isolated from livers of mice fed a regular diet (RD) or an HFD for 16 wk and labeled as in Fig. 6_D_ were incubated with endosomes labeled with Texas-red asialoglycoprotein, and fusion was quantified. Values are expressed as percentage of fusion events relative to total number of particles in field and are means +
se
of 4 different experiments. *P < 0.05. B, C) Homogenates (H), APGs, autophagolysosomes (APGL), and lysosomes (Lys) isolated from 6-h-starved (B and S in C) or fed (F) mice maintained on an RD or HFD were subjected to immunoblots for the indicated proteins. Arrow indicates CD-M6PR; other bands result from nonspecific cross-reaction.
Similar articles
- Cadmium-induced cytotoxicity in mouse liver cells is associated with the disruption of autophagic flux via inhibiting the fusion of autophagosomes and lysosomes.
Zou H, Wang T, Yuan J, Sun J, Yuan Y, Gu J, Liu X, Bian J, Liu Z. Zou H, et al. Toxicol Lett. 2020 Mar 15;321:32-43. doi: 10.1016/j.toxlet.2019.12.019. Epub 2019 Dec 17. Toxicol Lett. 2020. PMID: 31862506 - Annexin A5 stimulates autophagy and inhibits endocytosis.
Ghislat G, Aguado C, Knecht E. Ghislat G, et al. J Cell Sci. 2012 Jan 1;125(Pt 1):92-107. doi: 10.1242/jcs.086728. Epub 2012 Jan 20. J Cell Sci. 2012. PMID: 22266906 - The relationship between Cd-induced autophagy and lysosomal activation in WRL-68 cells.
Meng SF, Mao WP, Wang F, Liu XQ, Shao LL. Meng SF, et al. J Appl Toxicol. 2015 Nov;35(11):1398-405. doi: 10.1002/jat.3114. Epub 2015 Jan 29. J Appl Toxicol. 2015. PMID: 25639782 - The coordination of membrane fission and fusion at the end of autophagosome maturation.
Yu S, Melia TJ. Yu S, et al. Curr Opin Cell Biol. 2017 Aug;47:92-98. doi: 10.1016/j.ceb.2017.03.010. Epub 2017 Apr 29. Curr Opin Cell Biol. 2017. PMID: 28463755 Free PMC article. Review. - Maturation of autophagic vacuoles in Mammalian cells.
Eskelinen EL. Eskelinen EL. Autophagy. 2005 Apr;1(1):1-10. doi: 10.4161/auto.1.1.1270. Epub 2005 Apr 28. Autophagy. 2005. PMID: 16874026 Review.
Cited by
- Autophagy and aging.
Martinez-Lopez N, Athonvarangkul D, Singh R. Martinez-Lopez N, et al. Adv Exp Med Biol. 2015;847:73-87. doi: 10.1007/978-1-4939-2404-2_3. Adv Exp Med Biol. 2015. PMID: 25916586 Free PMC article. Review. - Transcription factor EB: a central regulator of both the autophagosome and lysosome.
Zhao E, Czaja MJ. Zhao E, et al. Hepatology. 2012 May;55(5):1632-4. doi: 10.1002/hep.25619. Hepatology. 2012. PMID: 22517549 Free PMC article. - Regulated degradation of Chk1 by chaperone-mediated autophagy in response to DNA damage.
Park C, Suh Y, Cuervo AM. Park C, et al. Nat Commun. 2015 Apr 16;6:6823. doi: 10.1038/ncomms7823. Nat Commun. 2015. PMID: 25880015 Free PMC article. - Chaperones in autophagy.
Kaushik S, Cuervo AM. Kaushik S, et al. Pharmacol Res. 2012 Dec;66(6):484-93. doi: 10.1016/j.phrs.2012.10.002. Epub 2012 Oct 8. Pharmacol Res. 2012. PMID: 23059540 Free PMC article. Review. - Lipid-enriched diet rescues lethality and slows down progression in a murine model of VCP-associated disease.
Llewellyn KJ, Nalbandian A, Jung KM, Nguyen C, Avanesian A, Mozaffar T, Piomelli D, Kimonis VE. Llewellyn KJ, et al. Hum Mol Genet. 2014 Mar 1;23(5):1333-44. doi: 10.1093/hmg/ddt523. Epub 2013 Oct 24. Hum Mol Genet. 2014. PMID: 24158850 Free PMC article.
References
- Cuervo A. M. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14:70–77. - PubMed
- Jager S., Bucci C., Tanida I., Ueno T., Kominami E., Saftig P., Eskelinen E. L. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci. 2004;117:4837–4848. - PubMed
- Gonzalez-Polo R. A., Boya P., Pauleau A. L., Jalil A., Larochette N., Souquere S., Eskelinen E. L., Pierron G., Saftig P., Kroemer G. The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci. 2005;118:3091–3102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 DK041918/DK/NIDDK NIH HHS/United States
- R37 AG021904/AG/NIA NIH HHS/United States
- T32AG023475/AG/NIA NIH HHS/United States
- R01 AG021904/AG/NIA NIH HHS/United States
- DK041918/DK/NIDDK NIH HHS/United States
- T32 AG023475/AG/NIA NIH HHS/United States
- AG031782/AG/NIA NIH HHS/United States
- P01 AG031782/AG/NIA NIH HHS/United States
- AG021904/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous