Rod-derived cone viability factor for treating blinding diseases: from clinic to redox signaling - PubMed (original) (raw)
Rod-derived cone viability factor for treating blinding diseases: from clinic to redox signaling
Thierry Léveillard et al. Sci Transl Med. 2010.
Abstract
The identification of one mechanism that causes vision loss in inherited degenerative retinal disorders revealed a new signaling molecule that represents a potential therapy for these currently untreatable diseases. This protein, called rod-derived cone viability factor (RdCVF), maintains the function and consequently the viability of cone photoreceptor cells in the retina; mice that lack this factor exhibit a progressive loss of photoreceptor cells. The gene encoding RdCVF also encodes, by differential splicing, a second product that has characteristics of a thioredoxin-like enzyme and protects both photoreceptor cells and, more specifically, its interacting protein partner, the tau protein, against oxidative damage. This signaling pathway potentially links environmental insults to an endogenous neuroprotective response.
Conflict of interest statement
Conflicts of interest: T.L. and J-A.S. are patent-holders on the use of RdCVF and RdCVF2 for the treatment of retinal and neurological disease
Figures
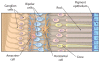
Fig. 1. Organization of the retina
CREDIT: C. BICKEL/SCIENCE TRANSLATIONAL MEDICINE
Fig. 2. Schematic representation of the Nxnl1 gene and its two products, RdCVF and RdCVFL
Alternative splicing generates the truncated trophic factor and the longer thioredoxin-like enzyme. In the ribbon diagrams, the thioredoxin catalytic site is shown in yellow, the shared portion of the thioredoxin fold in red, the additional thioredoxin fold (found only in RdCVFL) in green, and the RdCVF specific loops in blue. CREDIT: C. BICKEL/SCIENCE TRANSLATIONAL MEDICINE
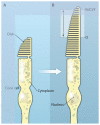
Fig. 3. Maintenance of the cone outer segment by RdCVF
RdCVF was injected into eyes of the P23H rat, a model of autosomal dominant RP. The cone cells (green) of the RdCVF-injected animal (bottom panel) display a longer outer segment (indicated by double arrows) and smaller diameter (asterisk) than those in the vehicle-injected rat (top panel). The relative positions of the cone outer segment in each case are indicated on the diagram on the right.
Similar articles
- Thioredoxin rod-derived cone viability factor protects against photooxidative retinal damage.
Elachouri G, Lee-Rivera I, Clérin E, Argentini M, Fridlich R, Blond F, Ferracane V, Yang Y, Raffelsberger W, Wan J, Bennett J, Sahel JA, Zack DJ, Léveillard T. Elachouri G, et al. Free Radic Biol Med. 2015 Apr;81:22-9. doi: 10.1016/j.freeradbiomed.2015.01.003. Epub 2015 Jan 14. Free Radic Biol Med. 2015. PMID: 25596499 - Metabolic and Redox Signaling of the Nucleoredoxin-Like-1 Gene for the Treatment of Genetic Retinal Diseases.
Clérin E, Marussig M, Sahel JA, Léveillard T. Clérin E, et al. Int J Mol Sci. 2020 Feb 27;21(5):1625. doi: 10.3390/ijms21051625. Int J Mol Sci. 2020. PMID: 32120883 Free PMC article. Review. - Restoration of Rod-Derived Metabolic and Redox Signaling to Prevent Blindness.
Clérin E, Aït-Ali N, Sahel JA, Léveillard T. Clérin E, et al. Cold Spring Harb Perspect Med. 2024 Nov 1;14(11):a041284. doi: 10.1101/cshperspect.a041284. Cold Spring Harb Perspect Med. 2024. PMID: 37848252 Review. - Viral-mediated RdCVF and RdCVFL expression protects cone and rod photoreceptors in retinal degeneration.
Byrne LC, Dalkara D, Luna G, Fisher SK, Clérin E, Sahel JA, Léveillard T, Flannery JG. Byrne LC, et al. J Clin Invest. 2015 Jan;125(1):105-16. doi: 10.1172/JCI65654. Epub 2014 Nov 21. J Clin Invest. 2015. PMID: 25415434 Free PMC article. - Maintaining Cone Function in Rod-Cone Dystrophies.
Sahel JA, Léveillard T. Sahel JA, et al. Adv Exp Med Biol. 2018;1074:499-509. doi: 10.1007/978-3-319-75402-4_62. Adv Exp Med Biol. 2018. PMID: 29721982 Review.
Cited by
- Prenatal Exposure to Curcumin Protects Rod Photoreceptors in a Transgenic Pro23His Swine Model of Retinitis Pigmentosa.
Scott PA, Kaplan HJ, McCall MA. Scott PA, et al. Transl Vis Sci Technol. 2015 Sep 16;4(5):5. doi: 10.1167/tvst.4.5.5. eCollection 2015 Sep. Transl Vis Sci Technol. 2015. PMID: 26396931 Free PMC article. - Cell Signaling with Extracellular Thioredoxin and Thioredoxin-Like Proteins: Insight into Their Mechanisms of Action.
Léveillard T, Aït-Ali N. Léveillard T, et al. Oxid Med Cell Longev. 2017;2017:8475125. doi: 10.1155/2017/8475125. Epub 2017 Sep 12. Oxid Med Cell Longev. 2017. PMID: 29138681 Free PMC article. Review. - Biocompatibility of a Conjugated Polymer Retinal Prosthesis in the Domestic Pig.
Maya-Vetencourt JF, Di Marco S, Mete M, Di Paolo M, Ventrella D, Barone F, Elmi A, Manfredi G, Desii A, Sannita WG, Bisti S, Lanzani G, Pertile G, Bacci ML, Benfenati F. Maya-Vetencourt JF, et al. Front Bioeng Biotechnol. 2020 Oct 15;8:579141. doi: 10.3389/fbioe.2020.579141. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33195139 Free PMC article. - In vivo measurement of mitochondrial ROS production in mouse models of photoreceptor degeneration.
Menger KE, Logan A, Luhmann UFO, Smith AJ, Wright AF, Ali RR, Murphy MP. Menger KE, et al. Redox Biochem Chem. 2023 Dec;5-6:None. doi: 10.1016/j.rbc.2023.100007. Redox Biochem Chem. 2023. PMID: 38046619 Free PMC article. - Rod metabolic demand drives progression in retinopathies.
Lin MK, Kim SH, Zhang L, Tsai YT, Tsang SH. Lin MK, et al. Taiwan J Ophthalmol. 2015 Jul-Sep;5(3):105-108. doi: 10.1016/j.tjo.2015.06.002. Epub 2015 Aug 25. Taiwan J Ophthalmol. 2015. PMID: 29018679 Free PMC article. Review.
References
- Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, Sun J, Redmond TM, Zhu X, Shindler KS, Ying GS, Ziviello C, Acerra C, Wright JF, McDonnell JW, High KA, Bennett J, Auricchio A. Gene Therapy for Leber’s Congenital Amaurosis is Safe and Effective Through 1.5 Years After Vector Administration. Mol Ther. 2009 Dec 1; [Epub ahead of print] - PMC - PubMed
- Hamel CP, Tsilou E, Pfeffer BA, Hooks JJ, Detrick B, Redmond TM. Molecular cloning and expression of RPE65, a novel retinal pigment epithelium-specific microsomal protein that is post-transcriptionally regulated in vitro. J Biol Chem. 1993;268:15751–15757. - PubMed
- Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, Claustres M, Hamel CP. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet. 1997;17:139–141. - PubMed
- Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, Gal A. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17:194–197. - PubMed
- Aguirre GD, Baldwin V, Pearce-Kelling S, Narfström K, Ray K, Acland GM. Congenital stationary night blindness in the dog: common mutation in the RPE65 gene indicates founder effect. Mol Vis. 1998;4:23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources