Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids - PubMed (original) (raw)
Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids
Yasemin Sancak et al. Cell. 2010.
Abstract
The mTORC1 kinase promotes growth in response to growth factors, energy levels, and amino acids, and its activity is often deregulated in disease. The Rag GTPases interact with mTORC1 and are proposed to activate it in response to amino acids by promoting mTORC1 translocation to a membrane-bound compartment that contains the mTORC1 activator, Rheb. We show that amino acids induce the movement of mTORC1 to lysosomal membranes, where the Rag proteins reside. A complex encoded by the MAPKSP1, ROBLD3, and c11orf59 genes, which we term Ragulator, interacts with the Rag GTPases, recruits them to lysosomes, and is essential for mTORC1 activation. Constitutive targeting of mTORC1 to the lysosomal surface is sufficient to render the mTORC1 pathway amino acid insensitive and independent of Rag and Ragulator, but not Rheb, function. Thus, Rag-Ragulator-mediated translocation of mTORC1 to lysosomal membranes is the key event in amino acid signaling to mTORC1.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
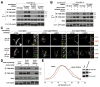
Figure 1. mTORC1 localizes to lysosomal membranes in an amino acid-dependent fashion while the Rag GTPases are constitutively localized to the same compartment.
(A) Images of HEK-293T cells co-immunostained for lysosomal protein LAMP2 (green) and mTOR (red). Cells were starved of and restimulated with amino acids for the indicated times before processing and imaging. (B) Images of HEK-293T cells co-immunostained for LAMP2 (green) and raptor (red) Cells were treated and processed as in (A). (C) Images of HEK-293T cells co-immunostained for LAMP2 (green) and RagC (red). Cells were treated and processed as in (A). (D) RagC interacts with RagA and RagB independently of amino acid availability. RagC-immunoprecipitates were prepared from HEK-293T cells starved or stimulated with amino acids as in (A), and immunoprecipitates and lysates were analyzed by immunoblotting for the indicated proteins. (E) Images of HEK-293T cells co-immunostained for RagA/B (green) and LAMP2 (red). Cells were treated, processed, and imaged as in (A). (F) GFP-RagB and GFP-RagBGTP co-localize with co-expressed LAMP1-mRFP independently of amino acid availability. HEK-293T cells transfected with the indicated cDNAs were treated and processed as in (A). (G) GFP-RagD and GFP-RagDGTP co-localize with co-expressed LAMP1-mRFP independently of amino acid availability. HEK-293T cells transfected with the indicated cDNAs were treated and processed as in (A). In all images, insets show selected fields that were magnified five times and their overlays. Scale bar is 10 μm. See also Fig S1.
Figure 2. The trimeric Ragulator complex interacts and co-localizes with the Rag GTPases
(A) Schematic amino acid sequence alignment of human MP1, p14, and p18 and their corresponding Drosophila orthologs. (B) Recombinant epitope-tagged Ragulator co-immunoprecipitates recombinant RagB and RagD. Anti-FLAG immunoprecipitates were prepared from HEK-293T cells co-transfected with the indicated cDNAs in expression vectors and cell lysates and immunoprecipitates analyzed by immunoblotting for levels of indicated proteins. The * indicates the band corresponding to the metap2 protein as it has the same apparent molecular weight as HA-GST-RagB. (C) Recombinant Ragulator co-immunoprecipitates mTORC1 when it is co-expressed with the GTP-bound mutant of RagB. HEK-293T cells were co-transfected with the indicated cDNAs in expression vectors and analyzed as in (B). The * indicates the bands corresponding to metap2 as it has the same apparent molecular weight as HA-GST-RagB. (D) Recombinant Ragulator co-immunoprecipitates endogenous RagA, RagB, and RagC. HEK-293T cells were co-transfected with indicated cDNAs in expression vectors and anti-FLAG immunoprecipitates analyzed as in (B). (E) Recombinant RagB-RagD heterodimers co-immunoprecipitate endogenous p14, MP1, and p18. HEK-293T cells were co-transfected with indicated cDNAs in expression vectors and anti-FLAG immunoprecipitates analyzed as in (B). (F) Endogenous RagC co-immunoprecipitates endogenous p14 and MP1. Anti-RagC immunoprecipitates were prepared from HEK-293T cells and analyzed for the levels of the indicated proteins. (G) Amino acids do not regulate the amounts of endogenous MP1, p14, RagA, or RagB that co-immunoprecipitate with recombinant p18. p18-null cells (p18−/−) or p18-null cells stably expressing FLAG-p18 (p18rev) were starved for amino acids for 50 min or starved and restimulated with amino acids for 10 min. After in-cell cross-linking, anti-FLAG immunoprecipitates were prepared from cell lysates and analyzed for the levels of the indicated proteins by immunoblotting. (H) Amino acids do not affect the amounts of endogenous p14 and p18 that co-immunoprecipitate with endogenous RagA/B. HEK-293T cells were treated as in (G) and anti-RagA/B immunoprecipitates analyzed by immunoblotting for the indicated proteins. (I) Endogenous Ragulator co-immunoprecipitates with FLAG-RagB independently of amino acid availability and GTP-loading of RagB. HEK-293T cells stably expressing FLAG-RagB or FLAG-RagBGTP were starved and restimulated with amino acids as in (G) and anti-FLAG immunoprecipitates analyzed for the levels of indicated proteins. (J) The Rag GTPases co-localize with GFP-tagged p18. HEK-293T cells were transfected with a cDNA encoding p18-GFP, processed for immunostaining for endogenous RagA/B or RagC, and imaged for the RagA/B (red) or RagC (red) signal as well as for p18-GFP fluorescence (green). Note: not all cells express p18-GFP. In all images, insets show selected fields that were magnified five times and their overlays. Scale bar is 10 μm. See also Fig S2.
Figure 3. The Ragulator is necessary to localize the Rag GTPases and mTORC1 to lysosomal membranes
(A) Images of p14-null or p18-null cells or their respective controls co-immunostained for RagC (red) and LAMP2 (green). Cells were starved of and restimulated with amino acids for the indicated times before processing for the immunofluorescence assay and imaging. (B) Images of p14-null or p18-null cells or their respective controls co-immunostained for mTOR (red) and LAMP2 (green). Cells were treated and processed as in (A). (C) Co-localization of mRFP-RagB (red) with GFP-Mito (green) in cells expressing mitochondrially-localized p18. p18-null cells (p18−/−), or p18-null cells expressing wild type p18 (p18rev) or mitochondrially-localized p18 (p18mito), were transiently transfected with the indicated cDNAs in expression plasmids and imaged. In all images, insets show selected fields that were magnified five times and their overlays. Scale bar is 10 μm. See also Fig S3.
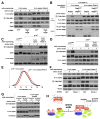
Figure 4. Ragulator-null and -depleted cells are highly deficient in the activation of mTORC1 signaling by amino acids
(A) p14 is necessary for the activation of the mTORC1 pathway by amino acids and serum. p14-null or control cells were starved of amino acids or serum for 50 minutes, or starved and re-stimulated with amino acids or serum for 10 minutes. Immunoblot analyses were used to measure the levels of the indicated proteins and phosphorylation states. (B) p18 is necessary for the activation of the mTORC1 pathway by amino acids and serum. p18-null or control cells were treated and analyzed as in (A). (C) Partial knockdown of MP1 blunts mTORC1 pathway activation by amino acids. HEK-293T cells expressing a control shRNA or two distinct shRNAs targeting MP1 were starved for amino acids for 50 minutes, or starved and stimulated with amino acids for 10 minutes and analyzed as in (A). (D) p14 and p18 are not necessary for mTORC2 pathway activity. p14-null or control cells were starved for serum, or starved and then re-stimulated with serum as in (A). p18-null or control cells were grown in complete media. Cell lysates were prepared and analyzed by immunobloting for the levels of Akt1 and Akt phosphorylation at the S473 site phosphorylated by mTORC2. (E) Decreased p14 expression impairs amino acid-induced mTORC1 activation in human cells. Cells derived from patients with lower p14 expression or healthy individuals were treated and analyzed as in (A). (F) Cells lacking Ragulator are smaller than control cells. Cell size distributions of p14-null or p18-null cells are overlaid with those from corresponding control cells. (G) Ragulator function is conserved in Drosophila cells. Drosophila S2 cells were transfected with a control dsRNA, or dsRNAs targeting dRagC, dMP1, dp14, or dp18, starved of amino acids for 90 minutes, or starved and restimulated with amino acids for 30 minutes. Levels of indicated proteins and phosphorylation states were analyzed by immunobloting. See also Fig S4.
Figure 5. In cells expressing raptor variants fused to the targeting signals of Rheb1 or Rap1b, mTORC1 localizes to lysosomal membranes in an amino acid-independent fashion
(A) Schematic of raptor fusion proteins that target mTORC1 to lysosomal membranes (raptor-Rheb15; raptor-Rap1b17) or to the plasma membrane (Raptor-HRas25) as well as proteins used as controls (wild-type raptor; raptor-Rheb15 CAAX). (B) Images of amino acid starved or replete cells expressing lysosomally-targeted or control HA-tagged raptor proteins and co-immunostained for the HA epitope (red) and endogenous LAMP2 (green). HEK-293T cells were transfected with the indicated cDNAs, starved of and restimulated with amino acids for the indicated times, and processed in the immunofluorescence assay. (C) Images of amino acid starved or replete cells co-expressing myc-mTOR and the indicated raptor fusion proteins and co-immunostained for the myc epitope (green) and endogenous LAMP2 (red). HEK-293T cells were co-transfected with the indicated cDNAs and treated and processed as in (B). In all images, insets show selected fields that were magnified five times and their overlays. Scale bar is 10 μm.
Figure 6. Constitutive association of raptor with lysosomal membranes, but not the plasma membrane, is sufficient to make the mTORC1 pathway insensitive to amino acid starvation
(A) The mTORC1 pathway is not sensitive to amino acid starvation in cells that express lysosomally-targeted but not control raptor proteins. HEK-293T cells were co-transfected with the indicated cDNA expression plasmids and starved of amino acids for 50 minutes or starved and restimulated with amino acids for 10 minutes. Cell lysates and anti-FLAG-S6K1 immunoprecipitates were analyzed by immunobloting for the levels of the indicated proteins and phosphorylation states. (B) The mTORC1 pathway is sensitive to serum starvation and insulin stimulation in cells that express lysosomally-targeted as well as control raptor proteins. HEK-293E cells were co-transfected with the indicated cDNA expression plasmids, starved of amino acids for 50 minutes or starved and restimulated with amino acids for 10 minutes. Duplicate cultures were starved of serum for 50 minutes or starved and stimulated with insulin for 10 minutes. Cell lysates and anti-FLAG-S6K1 immunoprecipitates were analyzed by immunobloting for the levels of the indicated proteins and phosphorylation states. (C) Images of cells stably expressing FLAG-raptor, FLAG-raptor-Rheb15, or FLAG-raptor-HRas25 and co-immunostained for endogenous mTOR (green) and endogenous LAMP2 (red). HEK-293T cells stably expressing the indicated proteins were treated as in (A) for the indicated times before processing in the immunofluorescence assay. In all images, insets show selected fields that were magnified five times and their overlays. Scale bar is 10 μm. (D) Targeting of mTORC1 to the lysosomal but not the plasma membrane makes the mTORC1 pathway insensitive to amino acid starvation. HEK-293T cells stably expressing FLAG-raptor, FLAG-raptor-Rheb15, or FLAG-raptor-HRas25 were treated as in (A) and analyzed by immunoblotting for the levels of the indicated proteins and phosphorylation states. (E) Targeting of mTORC1 to the lysosomal membrane increases cell size and pathway activity in cells under normal growth conditions. Cell size distributions of cells that stably express FLAG-raptor or FLAG-raptor-rheb15 as well as immunoblot analyses of the mTORC1 pathway in the same cells.
Figure 7. Targeting of mTORC1 to the lysosomal surface makes the activity of the mTORC1 pathway independent of Rag and Ragulator, but not, Rheb function
(A) In cells that express FLAG-raptor-Rheb15, mTORC1 pathway activity is independent of Rag GTPase function. Lysates of HEK-293T cells expressing FLAG-raptor or FLAG-raptor-Rheb15 were analyzed by immunobloting for the indicated proteins and phosphorylation states after disruption of Rag function by RNAi-mediated co-knockdown of RagA and RagB. Cells were starved of amino acids for 50 minutes or starved and restimulated with amino acids for 10 minutes before lysis. (B) In cells that express FLAG-raptor-Rheb15, mTORC1 pathway activity is independent of Rag GTPase function. Lysates of HEK-293T cells expressing FLAG-raptor or FLAG-raptor-Rheb15 were analyzed as in (A) after disruption of Rag function by expression of the dominant negative RagBGDP-RagDGTP heterodimer. Cells were treated and processed as in (A). (C) Stable expression of FLAG-raptor-Rheb15 but not FLAG-raptor in p14-null cells is sufficient to reactivate the mTORC1 pathway and make it insensitive to amino acid starvation. Cells stably expressing the indicated proteins were treated and analyzed as in (A). (D) Stable expression of FLAG-raptor-Rheb15 but not FLAG-raptor in p18-null cells is sufficient to reactivate the mTORC1 pathway and make it insensitive to amino acid starvation. Cells stably expressing the indicated proteins were treated and analyzed as in (A). (E) In p18-null cells expression of raptor-Rheb15, but not wild-type raptor, increases cell size. Cell size distributions of p18-null cells that stably express FLAG-raptor or FLAG-raptor-Rheb15. (F) In cells that express FLAG-raptor-Rheb15, the activity of the mTORC1 pathway is still Rheb-dependent. Lysates of HEK-293T cells that stably express FLAG-raptor or FLAG-raptor-Rheb15 were analyzed by immunobloting for the indicated proteins and phosphorylation states after disruption of Rheb function by an RNAi-mediated knockdown of Rheb1. Cells were treated as in (A). (G) Co-expression of plasma membrane-targeted raptor and plasma membrane-targeted Rheb1 renders the mTORC1 pathway insensitive to amino acid starvation. HEK-293T cells stably expressing the indicated proteins were treated and analyzed as in (A). (H) Model for amino-acid induced mTORC1 activation. In the absence of amino acids, mTORC1 cannot associate with the endomembrane system, and has no access to its activator Rheb. In the presence of amino acids, the Rag GTPases, which are tethered to the lysosomal surface by the Ragulator, serve as a docking site for mTORC1, allowing mTORC1 to associate with endomembranes and thus encounter and become activated by Rheb. See also Fig S5.
Comment in
- Lysosomal Rag-ulation of mTOR complex 1 activity.
Abraham RT. Abraham RT. Cell Metab. 2010 May 5;11(5):341-2. doi: 10.1016/j.cmet.2010.04.010. Cell Metab. 2010. PMID: 20444413 - Cell signalling: Of Rags and Ragulator.
Heinrichs A. Heinrichs A. Nat Rev Mol Cell Biol. 2010 Jun;11(6):388. doi: 10.1038/nrm2907. Epub 2010 May 6. Nat Rev Mol Cell Biol. 2010. PMID: 20445544 No abstract available. - Signalling: Of Rags and Ragulator.
Heinrichs A. Heinrichs A. Nat Rev Cancer. 2010 Jun;10(6):381. doi: 10.1038/nrc2863. Nat Rev Cancer. 2010. PMID: 20506585 No abstract available.
Similar articles
- Proton-assisted amino acid transporter PAT1 complexes with Rag GTPases and activates TORC1 on late endosomal and lysosomal membranes.
Ögmundsdóttir MH, Heublein S, Kazi S, Reynolds B, Visvalingam SM, Shaw MK, Goberdhan DC. Ögmundsdóttir MH, et al. PLoS One. 2012;7(5):e36616. doi: 10.1371/journal.pone.0036616. Epub 2012 May 4. PLoS One. 2012. PMID: 22574197 Free PMC article. - The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1.
Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. Sancak Y, et al. Science. 2008 Jun 13;320(5882):1496-501. doi: 10.1126/science.1157535. Epub 2008 May 22. Science. 2008. PMID: 18497260 Free PMC article. - Disruption of the Rag-Ragulator Complex by c17orf59 Inhibits mTORC1.
Schweitzer LD, Comb WC, Bar-Peled L, Sabatini DM. Schweitzer LD, et al. Cell Rep. 2015 Sep 1;12(9):1445-55. doi: 10.1016/j.celrep.2015.07.052. Epub 2015 Aug 20. Cell Rep. 2015. PMID: 26299971 Free PMC article. - Rag GTPase in amino acid signaling.
Kim J, Kim E. Kim J, et al. Amino Acids. 2016 Apr;48(4):915-928. doi: 10.1007/s00726-016-2171-x. Epub 2016 Jan 18. Amino Acids. 2016. PMID: 26781224 Review. - Amino acid signalling upstream of mTOR.
Jewell JL, Russell RC, Guan KL. Jewell JL, et al. Nat Rev Mol Cell Biol. 2013 Mar;14(3):133-9. doi: 10.1038/nrm3522. Epub 2013 Jan 30. Nat Rev Mol Cell Biol. 2013. PMID: 23361334 Free PMC article. Review.
Cited by
- Lysosomes and signaling pathways for maintenance of quiescence in adult neural stem cells.
Kobayashi T, Kageyama R. Kobayashi T, et al. FEBS J. 2021 May;288(10):3082-3093. doi: 10.1111/febs.15555. Epub 2020 Sep 15. FEBS J. 2021. PMID: 32902139 Free PMC article. Review. - RagC GTPase regulates mTOR to promote chemoresistance in senescence-like HepG2 cells.
Jiang W, Ou Z, Zhu Q, Zai H. Jiang W, et al. Front Physiol. 2022 Oct 4;13:949737. doi: 10.3389/fphys.2022.949737. eCollection 2022. Front Physiol. 2022. PMID: 36267578 Free PMC article. - Molecular logic of mTORC1 signalling as a metabolic rheostat.
Valvezan AJ, Manning BD. Valvezan AJ, et al. Nat Metab. 2019 Mar;1(3):321-333. doi: 10.1038/s42255-019-0038-7. Epub 2019 Mar 4. Nat Metab. 2019. PMID: 32694720 Review. - The crucial impact of lysosomes in aging and longevity.
Carmona-Gutierrez D, Hughes AL, Madeo F, Ruckenstuhl C. Carmona-Gutierrez D, et al. Ageing Res Rev. 2016 Dec;32:2-12. doi: 10.1016/j.arr.2016.04.009. Epub 2016 Apr 26. Ageing Res Rev. 2016. PMID: 27125853 Free PMC article. Review. - The brain as an insulin-sensitive metabolic organ.
Milstein JL, Ferris HA. Milstein JL, et al. Mol Metab. 2021 Oct;52:101234. doi: 10.1016/j.molmet.2021.101234. Epub 2021 Apr 15. Mol Metab. 2021. PMID: 33845179 Free PMC article. Review.
References
- Binda M, Péli-Gulli M, Bonfils G, Panchaud N, Urban J, Sturgill T, Loewith R, De Virgilio C. The Vam6 GEF controls TORC1 by activating the EGO complex. Mol Cell. 2009;35:563–573. - PubMed
- Bohn G, Allroth A, Brandes G, Thiel J, Glocker E, Schäffer AA, Rathinam C, Taub N, Teis D, Zeidler C, et al. A novel human primary immunodeficiency syndrome caused by deficiency of the endosomal adaptor protein p14. Nat Med. 2006;13:38–45. - PubMed
- Buerger C, DeVries B, Stambolic V. Localization of Rheb to the endomembrane is critical for its signaling function. Biochem Biophys Res Commun. 2006;344:869–880. - PubMed
- Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- CA103866/CA/NCI NIH HHS/United States
- DP2 ES032761/ES/NIEHS NIH HHS/United States
- R37 AI047389/AI/NIAID NIH HHS/United States
- R01 CA129105/CA/NCI NIH HHS/United States
- AI47389/AI/NIAID NIH HHS/United States
- R01 CA103866-01/CA/NCI NIH HHS/United States
- R01 AI047389-01/AI/NIAID NIH HHS/United States
- R01 AI047389/AI/NIAID NIH HHS/United States
- R01 CA103866/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous