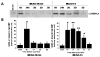Ubiquitin C-terminal hydrolase-L1 as a biomarker for ischemic and traumatic brain injury in rats - PubMed (original) (raw)
doi: 10.1111/j.1460-9568.2010.07097.x.
Linnet Akinyi, Dancia Scharf, Jixiang Mo, Stephen F Larner, Uwe Muller, Monika W Oli, Wenrong Zheng, Firas Kobeissy, Linda Papa, Xi-Chun Lu, Jitendra R Dave, Frank C Tortella, Ronald L Hayes, Kevin K W Wang
Affiliations
- PMID: 20384815
- PMCID: PMC2855688
- DOI: 10.1111/j.1460-9568.2010.07097.x
Ubiquitin C-terminal hydrolase-L1 as a biomarker for ischemic and traumatic brain injury in rats
Ming C Liu et al. Eur J Neurosci. 2010 Feb.
Abstract
Ubiquitin C-terminal hydrolase-L1 (UCH-L1), also called neuronal-specific protein gene product 9.5, is a highly abundant protein in the neuronal cell body and has been identified as a possible biomarker on the basis of a recent proteomic study. In this study, we examined whether UCH-L1 was significantly elevated in cerebrospinal fluid (CSF) following controlled cortical impact (CCI) and middle cerebral artery occlusion (MCAO; model of ischemic stroke) in rats. Quantitative immunoblots of rat CSF revealed a dramatic elevation of UCH-L1 protein 48 h after severe CCI and as early as 6 h after mild (30 min) and severe (2 h) MCAO. A sandwich enzyme-linked immunosorbent assay constructed to measure UCH-L1 sensitively and quantitatively showed that CSF UCH-L1 levels were significantly elevated as early as 2 h and up to 48 h after CCI. Similarly, UCH-L1 levels were also significantly elevated in CSF from 6 to 72 h after 30 min of MCAO and from 6 to 120 h after 2 h of MCAO. These data are comparable to the profile of the calpain-produced alphaII-spectrin breakdown product of 145 kDa biomarker. Importantly, serum UCH-L1 biomarker levels were also significantly elevated after CCI. Similarly, serum UCH-L1 levels in the 2-h MCAO group were significantly higher than those in the 30-min group. Taken together, these data from two rat models of acute brain injury strongly suggest that UCH-L1 is a candidate brain injury biomarker detectable in biofluid compartments (CSF and serum).
Figures
Fig. 1. Immunoblotting evidence of UCH-L1 release into rat CSF 48 h following controlled cortical impact TBI
A) Representative immunoblot results show presence of UCH-L1 protein in rat CSF 48h after TBI (control cortical impact) as compared to sham (48h) and naïve controls. B) Quantification indicates statistical significance (*p<0.05; n=3) from sham. C) Recombinant human UCH-L1 serial dilution and correlation to UCH-L1 band intensity (densitometric unit) by immunoblot.
Fig. 2. Immunoblotting evidence of UCH-L1 release into rat CSF following middle cerebral artery occlusion
A) Representative immunoblot results show significant elevated levels of UCH-L1 protein in rat CSF 6h after MCAO-30 min as compared to sham and 6h, 1D, 2D, and 3D after MCAO-2h. B) Quantification indicates statistical significance from sham (n=4 per time point) for MCAO-30 min and MCAO-2h (* p<0.05,**p<0.01).
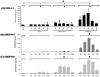
Fig. 3. UCH-L1 tissue specificity and brain region distribution
A) Immunoblot analyses of UCH-L1 protein levels in various rat tissues (n=3 pooled) and B) various rat brain regions (n=3 pooled); 15 μg protein loaded perlane.
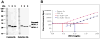
Fig. 4. UCH-L1 antibody and sandwich ELISA characterization
Sandwich ELISA set-up was described in Methods. The capture anti-UCH-L1 antibody was mouse monoclonal antibody; the detection antibody was an affinity purified HRP-labeled rabbit anti-UCH-L1 antibody; the antigen was recombinant human UCH-L1 (Boston Biochem). A) Both of the capture and detection antibodies detect recombinantUCH-L1 (lane 1) and single UCH-L1 protein band from total rat brain (lane 2) and human brain lysate (lane 3) respectively. B) Configured and optimized UCH-L1 sandwich ELISA standard curves (with UCH-L1 antigen) were performed in CSF (squares) and in serum (filled circles) (average + standard deviation shown). Three times standard deviation of background (no antigen) in CSF (triangle) and in serum (diamond) were also shown on Y-axis and plotted as dotted horizontal lines. Lowest detection limits calculated based on intercepts.
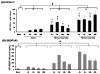
Fig. 5. Sandwich ELISA showing UCH-L1 elevation in rat CSF following controlled cortical impact TBI in comparison to αII-spectrin breakdown products(SBDP)
A) UCH-L1 levels in rat CSF. For UCH-L1, group comparison indicates that all groups (naïve, sham, CCI (1.0 mm), CCI (1.6 mm) all time points combined) are significantly different from each other (# p=0.01). UCH-L1 levels were significantly above sham for TBI (1.0 mm) and for TBI (1.6 mm) *p<0.05. Also, UCH-L1 levels were significantly above naïve for TBI (1.0 mm) and TBI (1.6 mm) §p<0.05). B) Calpain-generated SBDP145 levels in the same CSF samples (n=7) were also measured. For SBDP145, group comparison indicates that CCI (1.6 mm) (all time points combined), CCI (1.0 mm), sham and naïve groups are significantly different from each other(# p=0.001).
Fig. 6. Sandwich ELISA showing UCH-L1 elevation in rat CSF following MCAO in comparison to αII-spectrin breakdown products (SBDP)
A) UCH-L1 levels in rat CSF in naïve, sham, MCAO-30 min, and MCAO-2h. For UCH-L1, group comparison indicates that all groups (all time points combined; naïve, sham, MCAO-30 min and MCAO-2h) are significantly different from each other (# p=0.01). In comparisons with the counterparts such as sham and naïve there was significant evaluations of UCH-L1 (*p<0.05 and §p<0.05, respectively). B) Calpain-generated SBDP145 levels in the same CSF samples were also measured and group comparison indicates that MCAO-2h group (all time points combined) is significantly different from MCAO-30 min and sham groups (# p=0.001). C) Caspase-generated SBDP120 levels in the same CSF samples were similarly measured and group comparison indicates that both MCAO group-2h and MCAO-30 min groups (all time points combined) are significantly different from the sham group (# p=0.001).
Fig. 7. Sandwich ELISA shows UCH-L1 elevation in rat serum following controlled cortical impact TBI and MCAO
A) Control cortical Impact model (CCI). Group comparison indicates that CCI (1.6 mm) and sham groups (all time points combined) are significantly different (# p=0.001). By comparison of individual time points the UCH-L1 levels for select time points were above their sham counterparts: TBI (1.6 mm): *p<0.05). And in the comparison of UCH-L1 levels to naïve there were significance for sham (§p<0.05) highlighting the sensitivity of the marker to injury perturbation, and for TBI (1.6 mm: §p<0.05). B) MCAO model. Group comparison indicates that all groups (all time points combined) are significantly different (# p=0.001). By comparison of individual time points UCH-L1 levels for select time points were above their sham counterpoints for MCAO-30 min (*p<0.05)and for MCAO-2h (*p<0.05). For UCH-L1 levels for serum for both MCAO-30 min and -2h showed significance above naïve (§p<0.05).
Similar articles
- Different expression of ubiquitin C-terminal hydrolase-L1 and αII-spectrin in ischemic and hemorrhagic stroke: Potential biomarkers in diagnosis.
Ren C, Zoltewicz S, Guingab-Cagmat J, Anagli J, Gao M, Hafeez A, Li N, Cao J, Geng X, Kobeissy F, Mondello S, Larner SF, Hayes RL, Ji X, Ding Y. Ren C, et al. Brain Res. 2013 Dec 2;1540:84-91. doi: 10.1016/j.brainres.2013.09.051. Epub 2013 Oct 16. Brain Res. 2013. PMID: 24140110 - Acute Temporal Profiles of Serum Levels of UCH-L1 and GFAP and Relationships to Neuronal and Astroglial Pathology following Traumatic Brain Injury in Rats.
Huang XJ, Glushakova O, Mondello S, Van K, Hayes RL, Lyeth BG. Huang XJ, et al. J Neurotrauma. 2015 Aug 15;32(16):1179-89. doi: 10.1089/neu.2015.3873. Epub 2015 May 14. J Neurotrauma. 2015. PMID: 25763798 - Temporal response profiles of serum ubiquitin C-terminal hydrolase-L1 and the 145-kDa alpha II-spectrin breakdown product after severe traumatic brain injury in children.
Metzger RR, Sheng X, Niedzwecki CM, Bennett KS, Morita DC, Zielinski B, Schober ME. Metzger RR, et al. J Neurosurg Pediatr. 2018 Oct;22(4):369-374. doi: 10.3171/2018.4.PEDS17593. Epub 2018 Jun 29. J Neurosurg Pediatr. 2018. PMID: 29957142 - Serum ubiquitin C-terminal hydrolase L1 as a biomarker for traumatic brain injury: a systematic review and meta-analysis.
Li J, Yu C, Sun Y, Li Y. Li J, et al. Am J Emerg Med. 2015 Sep;33(9):1191-6. doi: 10.1016/j.ajem.2015.05.023. Epub 2015 May 29. Am J Emerg Med. 2015. PMID: 26087705 Review. - Ubiquitin C-terminal hydrolase-L1 (UCH-L1) as a therapeutic and diagnostic target in neurodegeneration, neurotrauma and neuro-injuries.
Wang KK, Yang Z, Sarkis G, Torres I, Raghavan V. Wang KK, et al. Expert Opin Ther Targets. 2017 Jun;21(6):627-638. doi: 10.1080/14728222.2017.1321635. Epub 2017 Apr 27. Expert Opin Ther Targets. 2017. PMID: 28434268 Review.
Cited by
- Bench-to-Bedside and Bedside Back to the Bench; Seeking a Better Understanding of the Acute Pathophysiological Process in Severe Traumatic Brain Injury.
Agoston DV. Agoston DV. Front Neurol. 2015 Mar 17;6:47. doi: 10.3389/fneur.2015.00047. eCollection 2015. Front Neurol. 2015. PMID: 25852631 Free PMC article. Review. - Predicting Clinical Outcomes 7-10 Years after Severe Traumatic Brain Injury: Exploring the Prognostic Utility of the IMPACT Lab Model and Cerebrospinal Fluid UCH-L1 and MAP-2.
Svingos AM, Robicsek SA, Hayes RL, Wang KK, Robertson CS, Brophy GM, Papa L, Gabrielli A, Hannay HJ, Bauer RM, Heaton SC. Svingos AM, et al. Neurocrit Care. 2022 Aug;37(1):172-183. doi: 10.1007/s12028-022-01461-y. Epub 2022 Mar 1. Neurocrit Care. 2022. PMID: 35229233 - Insights from Rodent Models for Improving Bench-to-Bedside Translation in Traumatic Brain Injury.
Pasam T, Dandekar MP. Pasam T, et al. Methods Mol Biol. 2024;2761:599-622. doi: 10.1007/978-1-0716-3662-6_40. Methods Mol Biol. 2024. PMID: 38427264 - Novel Mouse Tauopathy Model for Repetitive Mild Traumatic Brain Injury: Evaluation of Long-Term Effects on Cognition and Biomarker Levels After Therapeutic Inhibition of Tau Phosphorylation.
Rubenstein R, Sharma DR, Chang B, Oumata N, Cam M, Vaucelle L, Lindberg MF, Chiu A, Wisniewski T, Wang KKW, Meijer L. Rubenstein R, et al. Front Neurol. 2019 Mar 11;10:124. doi: 10.3389/fneur.2019.00124. eCollection 2019. Front Neurol. 2019. PMID: 30915013 Free PMC article. - Blood-based diagnostics of traumatic brain injuries.
Mondello S, Muller U, Jeromin A, Streeter J, Hayes RL, Wang KK. Mondello S, et al. Expert Rev Mol Diagn. 2011 Jan;11(1):65-78. doi: 10.1586/erm.10.104. Expert Rev Mol Diagn. 2011. PMID: 21171922 Free PMC article. Review.
References
- Berger RP, Adelson PD, Pierce MC, Dulani T, Cassidy LD, Kochanek PM. Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J Neurosurg. 2005;103(1 Suppl):61–68. - PubMed
- Berger RP, Beers SR, Richichi R, Wiesman D, Adelson PD. Serum biomarker concentrations and outcome after pediatric traumatic brain injury. J Neurotrauma. 2007;24:1793–1801. - PubMed
- Berger RP, Dulani T, Adelson PD, Leventhal JM, Richichi R, Kochanek PM. Identification of inflicted traumatic brain injury in well-appearing infants using serum and cerebrospinal markers: a possible screening tool. Pediatrics. 2006;117:325–332. - PubMed
- Choi DW. Exploratory clinical testing of neuroscience drugs. Nat Neurosci Suppl. 2002:1023–1025. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS049175-01 A1/NS/NINDS NIH HHS/United States
- R01 NS049175/NS/NINDS NIH HHS/United States
- R01 NS049175-02/NS/NINDS NIH HHS/United States
- R44 NS048685-03/NS/NINDS NIH HHS/United States
- 2R44NS048685-02/NS/NINDS NIH HHS/United States
- R44 NS048685/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous