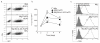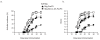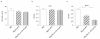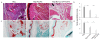FoxP3 and Bcl-xL cooperatively promote regulatory T cell persistence and prevention of arthritis development - PubMed (original) (raw)
FoxP3 and Bcl-xL cooperatively promote regulatory T cell persistence and prevention of arthritis development
Rizwanul Haque et al. Arthritis Res Ther. 2010.
Erratum in
- Arthritis Res Ther. 2012;14(2):401
Abstract
Introduction: Forkhead box p3 (FoxP3)-expressing regulatory T cells (Tregs) have been clearly implicated in the control of autoimmune disease in murine models. In addition, ectopic expression of FoxP3 conveys a Treg phenotype to CD4(+) T cells, lending itself to therapeutic use in the prevention of rheumatoid arthritis (RA). In this study, we generated therapeutically active Tregs with an increased life span and hence greater therapeutic potential.
Methods: We used retrovirus-mediated transduction to introduce FoxP3 or FoxP3 with anti-apoptotic Bcl-2 family molecule Bcl-xL linked by a 2A picornavirus self-cleaving peptide into CD4(+) T cells to generate Tregs. In addition, by using in vitro functional analyses and adoptive immunotherapy in a murine model of RA, we demonstrated that these Tregs were highly reactive.
Results: We found that CD4(+) T cells expressing both FoxP3 and Bcl-xL were able to differentiate into functional Tregs, which have a long-term survival advantage over cells transduced with FoxP3 alone. In an in vivo murine model, adoptive transfer of Tregs expressing both FoxP3 and Bcl-xL demonstrated more effective suppression of RA than CD4(+) T cells expressing FoxP3 alone.
Conclusions: FoxP3 and Bcl-xL can cooperatively promote the differentiation and persistence of Tregs, with the capacity to prevent arthritis. Our results provide a novel approach for generating highly reactive Tregs for augmenting cellular immunotherapy for autoimmune disease.
Figures
Figure 1
Expression of FoxP3 and Bcl-xL using a 2A gene sequence in primary CD4+ T cells. (a) Schematic representation of the retrovirus construct expressing Bcl-xL and FoxP3. ψ, packaging signal; 2A, picornavirus self-cleaving 2A sequence. (b) Naive CD4+ T cells from C57BL/6J mice were stimulated with anti-CD3 plus anti-CD28 antibodies. On day 2/3, T cells were transduced with retroviral vectors expressing green fluorescent protein (GFP) (Mig), GFP with FoxP3 (Mig-FoxP3), GFP with Bcl-xL (Mig-Bcl-xL), or GFP with Bcl-xL and FoxP3 (Mig-Bcl-xL-2A-FoxP3). On day 8 of primary culture, GFP+ CD4+ T cells were sorted, and protein expression of FoxP3, Bcl-xL, and β-actin was determined by Western blotting. Data are representative of three independent experiments. (c) Intracellular FoxP3 expression on day 6 of primary culture was analyzed by flow cytometry after gating on live CD4+ T cells. Data are representative of three independent experiments.
Figure 2
Retrovirus-mediated transduction of FoxP3 and Bcl-xL promotes survival of regulatory T cells in vitro. Naive CD4+ T cells from C57BL/6J mice were stimulated with anti-CD3 plus anti-CD28 antibodies, transduced on days 2/3 with retroviral vectors expressing green fluorescent protein (GFP), GFP with FoxP3, or GFP with Bcl-xL and FoxP3, and then recultured without any further stimulation. (a) Live CD4+ GFP+ T cells were visualized by flow cytometry on day 6 of culture. Data are representative of three independent experiments. (b) GFP+ T-cell recovery was normalized to take into account differences in initial transduction efficiency between cultures. Numbers of GFP+ cells present on day 2 were assigned a value of 100%, and numbers surviving on days 4, 6, and 8 were used to calculate the percentage recovery relative to day 2. Data represent the mean percentage change ± standard deviation from three separate experiments (*P <0.05, Student unpaired t test). (c) CD25 expression on day 4 of primary culture was analyzed by flow cytometry after gating on live GFP+ CD4+ T cells. Data are representative of three independent experiments.
Figure 3
Retrovirus-mediated transduction of FoxP3 and Bcl-xL enhances suppressive activity of regulatory T cells (Tregs) in vitro. Naive CD4+ T cells from C57BL/6J mice were stimulated with anti-CD3 plus anti-CD28 antibodies (Abs). On day 2/3, T cells were transduced with retroviral vectors expressing green fluorescent protein (GFP), GFP with FoxP3, or GFP with Bcl-xL and FoxP3. On day 6 of primary culture, GFP+ CD4+ Tregs were sorted and re-stimulated with anti-CD3 and anti-CD28 Abs (a) or co-cultured with naive CD4+ T cells from C57BL/6J mice (Tregs/T cells = 1:1) stimulated with anti-CD3 and anti-CD28 Abs for various periods (b, c). (a) Recall survival of Tregs, based on recovery of GFP+ CD4+ T cells over time. Cell numbers present on day 0 were assigned a value of 100%, and cell numbers surviving on day 2 to day 8 were used to calculate the percentage recovery. Data represent the mean percentage change ± standard deviation from three separate experiments. (b) Interleukin-2 (IL-2) production and interferon-gamma (IFN-γ) production were measured by enzyme-linked immunosorbent assay at 48 hours. Data are representative of three independent experiments. Proliferation on day 2 or 4 was analyzed by flow cytometry for 5-bromo-2-deoxyuridine (BrdU) incorporation (c) and thymidine incorporation during the last 12 hours (d). Data are representative of three experiments. *P <0.05, Student unpaired t test.
Figure 4
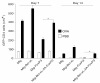
Retrovirus-mediated transduction of FoxP3 and Bcl-xL sustains survival of regulatory T cells in vivo. Naive CD4+ T cells from OT-II T-cell receptor transgenic mice were stimulated with peptide/antigen-presenting cells. On day 2/3, T cells were transduced with retroviral vectors expressing green fluorescent protein (GFP), GFP with Bcl-xL, GFP with FoxP3, or GFP with Bcl-xL and FoxP3. On day 6 of primary culture, 2 × 106 GFP+ CD4+ T cells were sorted and adoptively transferred into naive recipient mice that were subsequently challenged intraperitoneally with whole ovalbumin (OVA) protein (100 μg) in phosphate-buffered saline (PBS) (filled bars) or with PBS alone (open bars). On days 7 and 14, GFP+ CD4+ T cells were enumerated from pooled lymph nodes and spleen. Data are mean number of GFP+ CD4+ ± standard error of the mean from four individual mice and representative of three independent experiments (*P <0.05, Student unpaired t test).
Figure 5
Adoptive cell transfer of FoxP3- and Bcl-xL-transduced regulatory T cells suppresses collagen-induced arthritis (CIA). Naive CD4+ T cells from DBA/1J mice were stimulated with anti-CD3 plus anti-CD28 antibodies. On days 2 and 3, the cells were transduced with retroviral constructs: vector (Mig), FoxP3 (Mig-FoxP3), or FoxP3 with Bcl-xL (Mig-Bcl-xL-2A-FoxP3). On day 6, green fluorescent protein-positive (GFP+) T cells were sorted and prepared for adoptive cell transfer. CIA was induced in male DBA/1J mice (>4 months old) by one (day 0) intradermal immunization in the base of the tail with 100 μg of bovine type II collagen in complete Freund's adjuvant, containing 5 mg/mL killed Mycobacterium tuberculosis (H37Ra). On day 15 after the immunization, the mice received transduced GFP+ cells (2.5 × 106 per mouse, six mice per group). In the following days, the arthritis incidence (a) and clinical score (b) were evaluated by examining the paws and using a 4-point scale: 0, normal paw; 1, minimal swelling or redness; 2, redness and swelling involving the entire forepaw; 3, redness and swelling involving the entire limp; 4, joint deformity or ankylosis or both. Values are the mean ± standard error of the mean of data obtained in three experiments, and in each experiment, six mice per group were used. Summaries of the incidences and the mouse arthritis scores of the three experiments are listed in Additional files 1 and 2.
Figure 6
Adoptive cell transfer of FoxP3- and Bcl-xL-transduced regulatory T cells decreases arthritis-specific antibody production. The treatment of adoptive cell transfer for collagen-induced arthritis is described in the legend of Figure 5. On day 60 of immunization, sera were separated from all samples and the levels of serum total IgG and anti-bovine type II collagen antibodies were determined by enzyme-linked immunosorbent assay. (a) Total IgG. (b) IgG1. (c) IgG2a. Values are the mean ± standard error of the mean (n = 6). Data are representative of two similar experiments (*P <0.05, Student unpaired t test). OD, optical density.
Figure 7
Adoptive cell transfer of Bcl-xL- and FoxP3-transduced regulatory T cells reduces paw inflammation. The protocol of adoptive cell transfer for collagen-induced arthritis is described in the legend of Figure 5. On day 60 of immunization, hind foot paws were amputated, fixed, and decalcified. The tissues were embedded in paraffin, sectioned, and stained. (a) Hematoxylin and eosin (HE) staining. (b) HE semiquantitative score. (c) Safranin O-fast green staining. (d) Safranin O-fast green semiquantitative score. Data are representative of two similar experiments (*P <0.05, Student unpaired t test).
Comment in
- Bcl-xL affects the development of functional CD4 Tregs.
Sharabi A, Mozes E. Sharabi A, et al. Arthritis Res Ther. 2010;12(4):405. doi: 10.1186/ar3076. Epub 2010 Jul 23. Arthritis Res Ther. 2010. PMID: 20687901 Free PMC article. No abstract available.
Similar articles
- Efficient Therapeutic Function and Mechanisms of Human Polyclonal CD8+CD103+Foxp3+ Regulatory T Cells on Collagen-Induced Arthritis in Mice.
Sun J, Yang Y, Huo X, Zhu B, Li Z, Jiang X, Xie R, Gao L, Sun Y, Fan H, Zhu Y, Yang J. Sun J, et al. J Immunol Res. 2019 Feb 19;2019:8575407. doi: 10.1155/2019/8575407. eCollection 2019. J Immunol Res. 2019. PMID: 30915372 Free PMC article. - Collagen II-primed Foxp3 Transduced T Cells Ameliorate Collagen-induced Arthritis in Rats: The Effect of Antigenic Priming on T Regulatory Cell Function.
Zavvar M, Abdolmaleki M, Farajifard H, Noorbakhsh F, Azadmanesh K, Vojgani M, Nikcnam MH. Zavvar M, et al. Iran J Allergy Asthma Immunol. 2018 Aug 12;17(4):361-371. doi: 10.18502/ijaai.v17i4.95. Iran J Allergy Asthma Immunol. 2018. PMID: 30537799 - Daurinol Attenuates Autoimmune Arthritis via Stabilization of Nrp1-PTEN-Foxp3 Signaling in Regulatory T Cells.
Park MJ, Moon SJ, Lee EJ, Kim EK, Baek JA, Kim SY, Jung KA, Lee SH, Choi JW, Kim DS, Min JK, Park SH, Shin D, Cho ML. Park MJ, et al. Front Immunol. 2019 Jul 17;10:1526. doi: 10.3389/fimmu.2019.01526. eCollection 2019. Front Immunol. 2019. PMID: 31379809 Free PMC article. - Foxp3, Regulatory T Cell, and Autoimmune Diseases.
Tao JH, Cheng M, Tang JP, Liu Q, Pan F, Li XP. Tao JH, et al. Inflammation. 2017 Feb;40(1):328-339. doi: 10.1007/s10753-016-0470-8. Inflammation. 2017. PMID: 27882473 Review. - CD4+CD25+ regulatory T cells in autoimmune arthritis.
Oh S, Rankin AL, Caton AJ. Oh S, et al. Immunol Rev. 2010 Jan;233(1):97-111. doi: 10.1111/j.0105-2896.2009.00848.x. Immunol Rev. 2010. PMID: 20192995 Free PMC article. Review.
Cited by
- Cell-based therapies for rheumatoid arthritis: opportunities and challenges.
Li YJ, Chen Z. Li YJ, et al. Ther Adv Musculoskelet Dis. 2022 May 23;14:1759720X221100294. doi: 10.1177/1759720X221100294. eCollection 2022. Ther Adv Musculoskelet Dis. 2022. PMID: 35634355 Free PMC article. Review. - miR-142-3p regulates autophagy by targeting ATG16L1 in thymic-derived regulatory T cell (tTreg).
Lu Y, Gao J, Zhang S, Gu J, Lu H, Xia Y, Zhu Q, Qian X, Zhang F, Zhang C, Shen H, Hippen KL, Blazar BR, Lu L, Wang X. Lu Y, et al. Cell Death Dis. 2018 Feb 19;9(3):290. doi: 10.1038/s41419-018-0298-2. Cell Death Dis. 2018. PMID: 29459719 Free PMC article. - Manipulating regulatory T cells: a promising strategy to treat autoimmunity.
Zhang D, Tu E, Kasagi S, Zanvit P, Chen Q, Chen W. Zhang D, et al. Immunotherapy. 2015;7(11):1201-11. doi: 10.2217/imt.15.79. Epub 2015 Nov 16. Immunotherapy. 2015. PMID: 26568117 Free PMC article. Review. - Bcl-xL affects the development of functional CD4 Tregs.
Sharabi A, Mozes E. Sharabi A, et al. Arthritis Res Ther. 2010;12(4):405. doi: 10.1186/ar3076. Epub 2010 Jul 23. Arthritis Res Ther. 2010. PMID: 20687901 Free PMC article. No abstract available. - Programming of regulatory T cells from pluripotent stem cells and prevention of autoimmunity.
Haque R, Lei F, Xiong X, Bian Y, Zhao B, Wu Y, Song J. Haque R, et al. J Immunol. 2012 Aug 1;189(3):1228-36. doi: 10.4049/jimmunol.1200633. Epub 2012 Jun 25. J Immunol. 2012. PMID: 22732595 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials