Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression - PubMed (original) (raw)
Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression
R Bernard et al. Mol Psychiatry. 2011 Jun.
Abstract
Several studies have proposed that brain glutamate signaling abnormalities and glial pathology have a role in the etiology of major depressive disorder (MDD). These conclusions were primarily drawn from post-mortem studies in which forebrain brain regions were examined. The locus coeruleus (LC) is the primary source of extensive noradrenergic innervation of the forebrain and as such exerts a powerful regulatory role over cognitive and affective functions, which are dysregulated in MDD. Furthermore, altered noradrenergic neurotransmission is associated with depressive symptoms and is thought to have a role in the pathophysiology of MDD. In the present study we used laser-capture microdissection (LCM) to selectively harvest LC tissue from post-mortem brains of MDD patients, patients with bipolar disorder (BPD) and from psychiatrically normal subjects. Using microarray technology we examined global patterns of gene expression. Differential mRNA expression of select candidate genes was then interrogated using quantitative real-time PCR (qPCR) and in situ hybridization (ISH). Our findings reveal multiple signaling pathway alterations in the LC of MDD but not BPD subjects. These include glutamate signaling genes, SLC1A2, SLC1A3 and GLUL, growth factor genes FGFR3 and TrkB, and several genes exclusively expressed in astroglia. Our data extend previous findings of altered glutamate, astroglial and growth factor functions in MDD for the first time to the brainstem. These findings indicate that such alterations: (1) are unique to MDD and distinguishable from BPD, and (2) affect multiple brain regions, suggesting a whole-brain dysregulation of such functions.
Figures
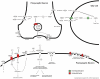
Figure 1
Synaptic location of elements of canonical glutamate signaling pathway. Model of glutamate synapse consisting of pre- and postsynaptic neuron and glial cell depicting the cellular location of key glutamate signaling and interacting proteins according the IPA knowledge base. Marked in green are gene products that are downregulated, whereas highlighted in red are upregulated gene products in the LC of MDD patients. Abbreviations: CALM – calmodulin; HOMER – homer homolog; Gβγ – beta/gamma subunit of G protein coupled receptor; GLNS – glutamine synthetase; GLS – glutaminase; GRIA – glutamate receptor, ionotropic AMPA; GRID – glutamate receptor, ionotropic, delta; GRIK – glutamate receptor, ionotropic, kainite; GRIN - glutamate receptor, ionotropic, NMDA; GRIP – glutamate receptor binding protein; GRM –glutamate receptor, metabotropic; PICK1 – protein interacting with C kinase 1; PSD-95 – postsynaptic density protein 95; SLC – member of solute carrier family
Similar articles
- Elevated gene expression of glutamate receptors in noradrenergic neurons from the locus coeruleus in major depression.
Chandley MJ, Szebeni A, Szebeni K, Crawford JD, Stockmeier CA, Turecki G, Kostrzewa RM, Ordway GA. Chandley MJ, et al. Int J Neuropsychopharmacol. 2014 Oct;17(10):1569-78. doi: 10.1017/S1461145714000662. Epub 2014 Jun 13. Int J Neuropsychopharmacol. 2014. PMID: 24925192 - Gene expression deficits in pontine locus coeruleus astrocytes in men with major depressive disorder.
Chandley MJ, Szebeni K, Szebeni A, Crawford J, Stockmeier CA, Turecki G, Miguel-Hidalgo JJ, Ordway GA. Chandley MJ, et al. J Psychiatry Neurosci. 2013 Jul;38(4):276-84. doi: 10.1503/jpn.120110. J Psychiatry Neurosci. 2013. PMID: 23415275 Free PMC article. - Glutamate transporters: a key piece in the glutamate puzzle of major depressive disorder.
Medina A, Burke S, Thompson RC, Bunney W Jr, Myers RM, Schatzberg A, Akil H, Watson SJ. Medina A, et al. J Psychiatr Res. 2013 Sep;47(9):1150-6. doi: 10.1016/j.jpsychires.2013.04.007. Epub 2013 May 24. J Psychiatr Res. 2013. PMID: 23706640 - Causes, consequences, and cures for neuroinflammation mediated via the locus coeruleus: noradrenergic signaling system.
Feinstein DL, Kalinin S, Braun D. Feinstein DL, et al. J Neurochem. 2016 Oct;139 Suppl 2:154-178. doi: 10.1111/jnc.13447. Epub 2016 Mar 10. J Neurochem. 2016. PMID: 26968403 Review. - Glial glutamate transporters: new actors in brain signaling.
López-Bayghen E, Ortega A. López-Bayghen E, et al. IUBMB Life. 2011 Oct;63(10):816-23. doi: 10.1002/iub.536. Epub 2011 Sep 7. IUBMB Life. 2011. PMID: 21901813 Review.
Cited by
- A brief history of the development of antidepressant drugs: from monoamines to glutamate.
Hillhouse TM, Porter JH. Hillhouse TM, et al. Exp Clin Psychopharmacol. 2015 Feb;23(1):1-21. doi: 10.1037/a0038550. Exp Clin Psychopharmacol. 2015. PMID: 25643025 Free PMC article. Review. - The implication of a diversity of non-neuronal cells in disorders affecting brain networks.
Carrier M, Dolhan K, Bobotis BC, Desjardins M, Tremblay MÈ. Carrier M, et al. Front Cell Neurosci. 2022 Nov 11;16:1015556. doi: 10.3389/fncel.2022.1015556. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36439206 Free PMC article. Review. - Evaluation of the Correlation Between Peripheral Inflammatory Markers and Suicide Risk in Drug-Naive First-Episode Schizophrenia.
Yeşilkaya ÜH, Şen M, Balcıoğlu YH, Gökçay H, Çelikkıran P, Balcıoğlu SK, Karamustafalıoğlu N. Yeşilkaya ÜH, et al. Noro Psikiyatr Ars. 2024 Aug 20;61(3):275-280. doi: 10.29399/npa.28663. eCollection 2024. Noro Psikiyatr Ars. 2024. PMID: 39258132 Free PMC article. - Altered functional protein networks in the prefrontal cortex and amygdala of victims of suicide.
Kékesi KA, Juhász G, Simor A, Gulyássy P, Szegő EM, Hunyadi-Gulyás E, Darula Z, Medzihradszky KF, Palkovits M, Penke B, Czurkó A. Kékesi KA, et al. PLoS One. 2012;7(12):e50532. doi: 10.1371/journal.pone.0050532. Epub 2012 Dec 6. PLoS One. 2012. PMID: 23272063 Free PMC article. - Astrocytes in Bipolar Disorder.
Butt AM, Rivera AD. Butt AM, et al. Adv Neurobiol. 2021;26:95-113. doi: 10.1007/978-3-030-77375-5_5. Adv Neurobiol. 2021. PMID: 34888832
References
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. - PubMed
- Rivelli S, Jiang W. Depression and ischemic heart disease: what have we learned from clinical trials? Curr Opin Cardiol. 2007;22(4):286–291. - PubMed
- Stimmel GL, Dopheide JA, Stahl SM. Mirtazapine: an antidepressant with noradrenergic and specific serotonergic effects. Pharmacotherapy. 1997;17(1):10–21. - PubMed
- Wong EH, Sonders MS, Amara SG, Tinholt PM, Piercey MF, Hoffmann WP, et al. Reboxetine: a pharmacologically potent, selective, and specific norepinephrine reuptake inhibitor. Biol Psychiatry. 2000;47(9):818–829. - PubMed
- Berman RM, Narasimhan M, Miller HL, Anand A, Cappiello A, Oren DA, et al. Transient depressive relapse induced by catecholamine depletion: potential phenotypic vulnerability marker? Arch Gen Psychiatry. 1999;56(5):395–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- L99MH60398/MH/NIMH NIH HHS/United States
- 1K99MH081927-01A1/MH/NIMH NIH HHS/United States
- P50 MH060398/MH/NIMH NIH HHS/United States
- R00 MH081927/MH/NIMH NIH HHS/United States
- K99 MH081927/MH/NIMH NIH HHS/United States