Mechanical control of tissue and organ development - PubMed (original) (raw)
Review
Mechanical control of tissue and organ development
Tadanori Mammoto et al. Development. 2010 May.
Abstract
Many genes and molecules that drive tissue patterning during organogenesis and tissue regeneration have been discovered. Yet, we still lack a full understanding of how these chemical cues induce the formation of living tissues with their unique shapes and material properties. Here, we review work based on the convergence of physics, engineering and biology that suggests that mechanical forces generated by living cells are as crucial as genes and chemical signals for the control of embryological development, morphogenesis and tissue patterning.
Figures
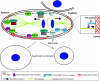
Fig. 1.
Regulation of asymmetric cell division by external physical cues in C. elegans eggs. In the C. elegans egg, contact with other cells causes the local accumulation of PAC-1 RhoGAP, which inhibits CDC-42 GTPase activity, leading to PAR-6 accumulation with activated CDC-42 at sites free of cell-cell contact. In addition, sperm donates the CYK-4 RhoGAP to the egg, which induces the formation of a loose actin network domain around the entry site (potential posterior pole) that generates a contractile cortical actin flow towards the opposite site (anterior pole). This cortical actin flow moves the PAR-3/6 complex to the anterior pole, and the cytoplasmic PAR-2 to the posterior pole. This PAR polarity modulates the pulling force generated by dynein-dynactin motor complexes that is exerted on depolymerizing microtubules through the LIN-5–GRP-1/2–Gα complex to determine spindle orientation (see inset).
Fig. 2.
Mechanical control of cell sorting and gastrulation. (A) Progenitor cell sorting. Different types of cells (depicted as light blue and green) sort and aggregate depending on the level of traction forces exerted on cell surface cadherins. Tensional forces generated by actomyosin interactions in the cortical cytoskeleton, which are controlled by TGFβ/Nodal signaling and exerted on cadherins, play a crucial role in cell sorting. G protein-coupled receptors and p120 catenin control the assembly of cadherins and cortical actin. (B) Axis formation. As mediolateral intercalation begins, cells exert traction on adjacent cells in the mediolateral direction through actin-based filopodia at cell-cell junctions. These tensional forces, which are regulated by the Wnt/planar cell polarity (PCP) and Rho/Rho-associated kinase (ROCK) signaling pathways, stabilize the junctions and drive cell rearrangements. Pulling by neighboring cells shortens the cell aggregate in the mediolateral direction (convergence) and extends it in the anteroposterior direction (extension). (C) Tissue folding. In Drosophila, the mechanical compression of cells due to germ band extension through cell intercalation within a limiting boundary induces the expression of the gene encoding the basic helix-loop-helix transcription factor Twist through β-catenin nuclear translocation. This causes the rearrangement of cortical actin and cell-cell junctions, which drives apical constriction through the secreted protein Folded gastrulation (Fog), the zinc-finger protein Snail and ROCK. This, in turn, results in ventral furrow formation. Morphogens (e.g. BMPs, Wnt and Shh) modulate this signaling mechanism. (D) Dorsal closure. The migrating cells at the leading edge of the Drosophila dorsal epidermis extend actin-based filopodia that promote the formation of new cell-cell junctions when they contact cells on the opposing leading edge. Cytoskeletal tensional forces exerted on, or transmitted across, adhesions under the control of the Wnt/PCP and Rho/ROCK signaling pathways might help to drive tissue closure; underlying amnioserosa cells also contribute by mechanically pulling on the overlying epidermal cells. Although mechanically driven, the entire process of dorsal closure is controlled by soluble morphogens, such as Dpp.
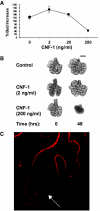
Fig. 3.
Cytoskeletal tension and ECM mechanics in lung development. (A) Graph showing the effects of the Rho activator and tension promoter, cytotoxic necrotizing factor 1 (CNF-1), on epithelial branch formation during mouse lung development. Note that low doses of CNF-1 (2 and 20 ng/ml) increase terminal bud number, whereas a high dose (200 ng/ml), which results in high levels of tension in the growing cells, inhibits this process. (B) Photomicrographs of lung rudiments cultured for two days with or without 2 or 200 ng/ml CNF-1. There is an increase in distal lung buds at the low dose and a large-scale contraction of the entire gland at the high CNF-1 dose that greatly enhances cell contractility. Scale bar: 500 μm. (C) Immunofluorescence micrograph of a section through a normal lung rudiment stained for the basement membrane protein laminin. The arrow indicates a region of the basement membrane at the periphery of one epithelial bud, showing that the thinnest regions of the basement membrane typically appose the part of the epithelium with the most rapid cell growth and tissue expansion rates. Reproduced, with permission, from Moore et al. (Moore et al., 2005).
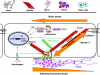
Fig. 4.
Model of the mechanical control of cell fate switching. Mechanical forces generated by acto-myosin interactions within the cytoskeleton are resisted by integrin adhesions to the ECM, cadherin adhesions to neighboring cells and internal cytoskeletal struts (e.g. microtubules and cross-linked actin bundles as in filopodia), thereby establishing a tensional prestress that stabilizes cell and tissue structure through a tensegrity mechanism (reviewed by Ingber, 2006). Alterations in the mechanical forces that are balanced between the ECM, neighboring cells and opposing cytoskeletal elements modulate intracellular biochemistry and gene expression (Ingber, 2006; Stamenovic and Ingber, 2009). Molecules involved in cytoskeletal tension generation, including actin, myosin II, Rho, ROCK and the Rho modulator p190RhoGAP, play a central role in this form of mechanical signaling. External forces (e.g. fluid shear stress) also can modulate gene transcription, for example through changes in nitric oxide (NO) signaling. The binding of growth factors and ECM ligands to their respective cell surface receptors can alter cellular biochemistry and gene expression independently of changes in cell prestress or external forces; however, mechanical stresses govern the final biochemical response and determine cell fate (e.g. whether stem cells differentiate into bone, muscle, nerve, blood or other cells). Physical forces exerted on surface adhesion receptors are also transmitted directly to the nucleus along cytoskeletal filaments and molecules that connect the cytoskeleton to the nucleus, such as nesprin (Wang et al., 2009). Nuclear envelope molecules, such as lamin, stabilize nuclear architecture under mechanical strain, and defects in nuclear mechanical signaling can lead to developmental abnormalities.
Similar articles
- Developing pressures: fluid forces driving morphogenesis.
Navis A, Bagnat M. Navis A, et al. Curr Opin Genet Dev. 2015 Jun;32:24-30. doi: 10.1016/j.gde.2015.01.010. Epub 2015 Feb 17. Curr Opin Genet Dev. 2015. PMID: 25698116 Free PMC article. Review. - Mechanobiology and developmental control.
Mammoto T, Mammoto A, Ingber DE. Mammoto T, et al. Annu Rev Cell Dev Biol. 2013;29:27-61. doi: 10.1146/annurev-cellbio-101512-122340. Annu Rev Cell Dev Biol. 2013. PMID: 24099083 Review. - [The irruption of mechanics in the chemistry of life].
Mège RM, Ladoux B. Mège RM, et al. Med Sci (Paris). 2018 Nov;34(11):963-971. doi: 10.1051/medsci/2018241. Epub 2018 Dec 10. Med Sci (Paris). 2018. PMID: 30526840 Review. French. - The mechanics of development: Models and methods for tissue morphogenesis.
Gjorevski N, Nelson CM. Gjorevski N, et al. Birth Defects Res C Embryo Today. 2010 Sep;90(3):193-202. doi: 10.1002/bdrc.20185. Birth Defects Res C Embryo Today. 2010. PMID: 20860059 Free PMC article. Review. - The interplay between cell signalling and mechanics in developmental processes.
Miller CJ, Davidson LA. Miller CJ, et al. Nat Rev Genet. 2013 Oct;14(10):733-44. doi: 10.1038/nrg3513. Nat Rev Genet. 2013. PMID: 24045690 Free PMC article. Review.
Cited by
- Integrin-dependent force transmission to the extracellular matrix by α-actinin triggers adhesion maturation.
Roca-Cusachs P, del Rio A, Puklin-Faucher E, Gauthier NC, Biais N, Sheetz MP. Roca-Cusachs P, et al. Proc Natl Acad Sci U S A. 2013 Apr 9;110(15):E1361-70. doi: 10.1073/pnas.1220723110. Epub 2013 Mar 20. Proc Natl Acad Sci U S A. 2013. PMID: 23515331 Free PMC article. - Kinase-interacting substrate screening is a novel method to identify kinase substrates.
Amano M, Hamaguchi T, Shohag MH, Kozawa K, Kato K, Zhang X, Yura Y, Matsuura Y, Kataoka C, Nishioka T, Kaibuchi K. Amano M, et al. J Cell Biol. 2015 Jun 22;209(6):895-912. doi: 10.1083/jcb.201412008. J Cell Biol. 2015. PMID: 26101221 Free PMC article. - Scanning Probe Microscopies: Imaging and Biomechanics in Reproductive Medicine Research.
Andolfi L, Battistella A, Zanetti M, Lazzarino M, Pascolo L, Romano F, Ricci G. Andolfi L, et al. Int J Mol Sci. 2021 Apr 7;22(8):3823. doi: 10.3390/ijms22083823. Int J Mol Sci. 2021. PMID: 33917060 Free PMC article. Review. - Organotypic liver culture models: meeting current challenges in toxicity testing.
LeCluyse EL, Witek RP, Andersen ME, Powers MJ. LeCluyse EL, et al. Crit Rev Toxicol. 2012 Jul;42(6):501-48. doi: 10.3109/10408444.2012.682115. Epub 2012 May 15. Crit Rev Toxicol. 2012. PMID: 22582993 Free PMC article. Review. - Piezo2 expressed in proprioceptive neurons is essential for skeletal integrity.
Assaraf E, Blecher R, Heinemann-Yerushalmi L, Krief S, Carmel Vinestock R, Biton IE, Brumfeld V, Rotkopf R, Avisar E, Agar G, Zelzer E. Assaraf E, et al. Nat Commun. 2020 Jun 23;11(1):3168. doi: 10.1038/s41467-020-16971-6. Nat Commun. 2020. PMID: 32576830 Free PMC article.
References
- Ash J. F., Spooner B. S., Wessells N. K. (1973). Effects of papaverine and calcium-free medium on salivary gland morphogenesis. Dev. Biol. 33, 463-469 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources