The Cullin 3 substrate adaptor KLHL20 mediates DAPK ubiquitination to control interferon responses - PubMed (original) (raw)
The Cullin 3 substrate adaptor KLHL20 mediates DAPK ubiquitination to control interferon responses
Yu-Ru Lee et al. EMBO J. 2010.
Abstract
Death-associated protein kinase (DAPK) was identified as a mediator of interferon (IFN)-induced cell death. How IFN controls DAPK activation remains largely unknown. Here, we identify the BTB-Kelch protein KLHL20 as a negative regulator of DAPK. KLHL20 binds DAPK and Cullin 3 (Cul3) via its Kelch-repeat domain and BTB domain, respectively. The KLHL20-Cul3-ROC1 E3 ligase complex promotes DAPK polyubiquitination, thereby inducing the proteasomal degradation of DAPK. Accordingly, depletion of KLHL20 diminishes DAPK ubiquitination and degradation. The KLHL20-mediated DAPK ubiquitination is suppressed in cells receiving IFN-alpha or IFN-gamma, which induces an enrichment/sequestration of KLHL20 in the PML nuclear bodies, thereby separating KLHL20 from DAPK. Consequently, IFN triggers the stabilization of DAPK. This mechanism of DAPK stabilization is crucial for determining IFN responsiveness of tumor cells and contributes to IFN-induced autophagy. This study identifies KLHL20-Cul3-ROC1 as an E3 ligase for DAPK ubiquitination and reveals a regulatory mechanism of DAPK, through blocking its accessibility to this E3 ligase, in IFN-induced apoptotic and autophagic death. Our findings may be relevant to the problem of IFN resistance in cancer therapy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
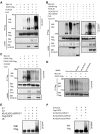
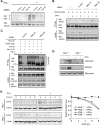
Interaction of KLHL20 with Cul3 and DAPK. (A) Identification of KLHL20 as a DAPK-binding protein. Yeast strain L40 contransformed with Gal- and LexA-based constructs as indicated was assayed for His3 phenotype (−His) or β-galactosidase activity (β-Gal). (B) Endogenous KLHL20 interacts with endogenous DAPK. Lysates of HeLa cells were used for immunoprecipitation with anti-DAPK antibody or control IgG and the immunoprecipitates and cell lysate were subjected to western blot with antibodies as indicated. (C) The DD of DAPK is involved in binding KLHL20. Lysates of 293T cells transfected with DAPK-Flag or DAPKΔDD-Flag were used for immunoprecipitation and western blot analyses with antibodies as indicated. (D, E) Mapping the KLHL20 domain responsible for binding DAPK (D) or Cul3 (E). 293T cells were cotransfected with various constructs as indicated. Cells were lysed for immunoprecipitation and western blot analyses with antibodies as indicated. The position of immunoglobulin heavy chain is marked with an asterisk. (F) Cul3 coprecipitates with DAPK. Lysates of 293T cells cotransfected with various constructs were analysed by immunoprecipitation and/or western blot with indicated antibodies. (G) KLHL20 mediates the interaction between DAPK and Cul3. HeLa cells were infected with lentivirus carrying indicated siRNA and then selected with puromycin. The resulting stable knockdown cells were analysed by immunoprecipitation followed by western blot with antibodies as indicated. KLHL20, Cul3, and DAPK expression levels were assayed by western blot (bottom panel).
Figure 2
KLHL20–Cul3–ROC1 complex is a ubiquitin ligase for DAPK. (A, B) KLHL20–Cul3–ROC1 complex promotes DAPK ubiquitination in vivo. 293T cells were transfected with constructs expressing Myc-ubiquitin (Myc-Ub), ROC1 (not indicated), Cul3 (or mutant), KLHL20 (or mutant), and/or DAPK-Flag and treated with MG132 for 16 h. Cell lysates were used to detect DAPK ubiquitination by immunoprecipitation with anti-Flag antibody, followed by western blot with anti-Myc antibody. The expression levels of Cul3 and KLHL20 were examined by western blot (bottom panel). (C) The DD of DAPK is important for DAPK ubiquitination by KLHL20–Cul3–ROC1 complex. 293T cells transfected with indicated constructs and treated with MG132 were assayed for DAPK ubiquitination as in (A). (D) Endogenous KLHL20 promotes DAPK ubiquitination. HeLa cell derivatives as in Figure 1G were transfected with Myc-Ub and/or DAPK-Flag and assayed for DAPK ubiquitination as in (A). (E–F) In vitro ubiquitination of DAPK by the KLHL20–Cul3–ROC1 E3 ligase. Cul3 complex was purified by Glutathione Sepharose from lysates of 293T cells transfected with GST-Cul3 (or its mutant), Myc-ROC1, and KLHL20-Myc (or its mutant). The copurification of ROC1 and/or KLHL20 with GST-Cul3 was shown in Supplementary Figure S5. The Cul3 complex was subjected to in vitro ubiquitination assay in the presence of E1, E2, ubiquitin, and/or Flag-DAPK purified from baculovirus (see Materials and methods). The reaction was resolved on SDS–PAGE and analysed by western blot with anti-Flag antibody.
Figure 3
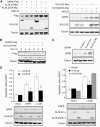
KLHL20 enhances DAPK proteasomal degradation and attenuates DAPK proapoptotic activity. (A) KLHL20 promotes proteasomal degradation of DAPK. 293T cells transfected with indicated constructs were treated with 1 μM MG132 or DMSO (−) for 12 h, and then lysed for western blot analyses. (B) KLHL20 promotes DAPK turnover. HeLa cells transfected with indicated constructs were treated with 100 μg/ml cycloheximide for various time points before lysis. Cell lysates were analysed by western blot. The amounts of Flag-DAPK relative to that of untreated cells are indicated. (C) Knockdown of KLHL20 increases DAPK steady-state level. HeLa cell derivatives as in Figure 1G were analysed for DAPK level by western blot. (D) KLHL20 inhibits the proapoptotic function of DAPK. NIH3T3 cells were transiently transfected with DAPK (or DAPKΔCaM) together with or without KLHL20 (or KLHL20ΔK). Apoptosis was assayed by the Cell Death Detection ELISA kit. Data are represented as mean±s.e.m. (*P<0.05; **P<0.005; _n_=3). The expression levels of various proteins are shown on the bottom panel.
Figure 4
IFN blocks KLHL20-dependent DAPK ubiquitination and degradation. (A) IFN upregulates DAPK. 293T cells transfected with DAPK-Flag were treated with 1000 U/ml IFN-γ for indicated time points and then lysed for western blot analysis. (B) IFN inhibits proteasomal degradation of DAPK. HeLa cells were treated with 1500 U/ml IFN-α or 1000 U/ml IFN-γ for 18 h and then with 30 μM MG132 (or DMSO) for 3 h. The level of endogenous DAPK was analysed by western blot. (C, D) IFN-α or IFN-γ blocks KLHL20-dependent DAPK ubiquitination. 293T cells were transfected with Myc-Ub, Cul3, ROC1, KLHL20, and/or DAPK-Flag. Two days after transfection, cells were treated with IFN-γ for indicated time points (C) or IFN-α for 18 h (D). DAPK ubiquitination was assayed as in Figure 2A.
Figure 5
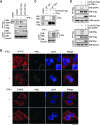
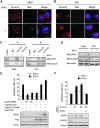
IFN triggers the disruption of KLHL20–DAPK complex by sequestrating KLHL20 in PML-NBs. (A) IFN blocks the interaction of KLHL20 with endogenous DAPK. HeLa cells transfected with KLHL20-Flag were treated with IFN-γ for 18 h and lysed for immunoprecipitation with anti-Flag antibody. The immunoprecipitates and cell lysates were analysed by western blot with indicated antibodies. (B) IFN triggers the enrichment of KLHL20 in PML-NBs. HeLa cells were treated with or without IFN-γ for 18 h. Cells were fixed, triple stained by DAPI, anti-KLHL20 antibody and anti-PML antibody (upper panel) or by DAPI, anti-DAPK antibody and anti-PML antibody (bottom panel), and examined by confocal microscopy. The box area was amplified to show the colocalization of KLHL20 and PML. Bar, 20 μm. (C) Interaction of KLHL20 with PML. HeLa cells transfected and treated as in (A) were lysed for immunoprecipitation with anti-Flag or a control antibody (IgG). The immunoprecipitates and cell lysates were analysed by western blot with antibodies as indicated. (D) DAPK and PML compete for binding KLHL20. 293T cells transfected with indicated constructs were subjected to immunoprecipitation with anti-Flag, followed by western blot with indicated antibodies.
Figure 6
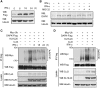
PML depletion reverses the inhibitory effects of IFN on DAPK ubiquitination and degradation. (A) Generation of PML knockdown cells. HeLa cells were infected with lentivirus carrying indicated siRNA and selected with puromycin. Cells were treated with or without IFN-γ for 18 h and then lysed for western blot analysis. (B) PML siRNA rescues the interaction between DAPK and KLHL20 in IFN-γ-treated cells. Cells as in (A) were transfected with KLHL20-Flag and treated with IFN-γ for 18 h. Cells were lysed for immunoprecipitation with anti-Flag antibody. The immunoprecipitates and cell lysates were analysed by western blot with antibodies as indicated. (C) PML siRNA rescues KLHL20-mediated DAPK ubiquitination in IFN-γ-treated cells. Cells as in (A) were transfected with indicated constructs and treated with IFN-γ for 18 h. DAPK ubiquitination was analysed as in Figure 2A. The expression levels of various proteins are shown on the bottom. (D) IFN-γ fails to upregulate DAPK in PML null cells. PML+/+ or PML−/− MEFs were treated with IFN-γ for 18 h and then lysed for western blot analysis with indicated antibodies. (E) PML is required for IFN-γ-induced DAPK stabilization. Cells as in (D) were treated with IFN-γ for 18 h and then with 50 μg/ml cycloheximide for indicated time points. Cells were lysed for western blot analysis and the level of DAPK was normalized to that of tubulin and plotted on the right.
Figure 7
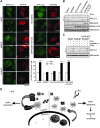
Blockage of KLHL20-mediated DAPK degradation contributes to MM cell responsiveness to IFN. (A, B) IFN-α induces PML-NBs and KLHL20 relocation in H929 cells but not in XG1 cells. Cells treated with IFN-α (1000 U/ml) for 16 h were fixed, stained by DAPI (blue), anti-KLHL20 antibody and anti-PML antibody, and examined by confocal microscopy. Bar, 10 μm. (C) IFN-α induces the disruption of KLHL20–DAPK complex in H929 cells but not in XG1 cells. Cells as indicated were treated as in (A) and then subjected to immunoprecipitation with anti-KLHL20 antibody or control antibody (IgG), followed by western blot with anti-KLHL20 or anti-DAPK antibody. (D) IFN-α induces the upregulation of DAPK in H929 cells but not in XG1 cells. Cells treated as in (A) were analysed by western blot with antibodies as indicated. (E) H929 cells were infected with lentivirus-expressing KLHL20, KLHL20m6 or DAPK siRNA and then selected with blasticidin (for KLHL20 construct) or puromycin (for siDAPK construct). To generate cells stably expressing both KLHL20 and DAPKΔDD, H929 cells were first infected with retrovirus carrying DAPKΔDD and selected with puromycin. The resulting stable line was then infected with lentivirus-expressing KLHL20 and selected with blasticidin. Cell lysates were analysed by western blot with antibodies as indicated (bottom panel). Alternatively, cells were treated with IFN-α and apoptosis was assayed by Annexin V staining followed by flow cytometry analysis (upper panel). (F) XG1 cells were infected with lentivirus carrying indicated siRNAs and then selected with hygromycin (for DAPK siRNA construct) and/or puromycin (for KLHL20 siRNA construct). Cells were lysed for western blot analysis (bottom panel). Alternatively, IFN-α-induced apoptosis was assayed as in (C). Data are represented as mean±s.e.m. (*P<0.05; **P<0.005; ***P<0.0005; _n_=3).
Figure 8
Blockage of KLHL20-mediated DAPK degradation contributes to IFN-induced autophagy. (A) MCF7-LC3 cells were transfected with various constructs together with mCherry-expressing construct and then treated with or without IFN-γ. The GFP-LC3 signal was examined by confocal microscopy (for image presentation) and epifluorescent microscopy (for quantification). Bar, 20 μm. The number of GFP-LC3 dots per cell was quantified from 50 mCherry-positive cells and plotted (bottom panel). Data are represented as mean±s.e.m. (***P<0.0005; _n_=3). (B) MCF7 parental cells were transfected and treated as in (A) and then analysed by western blot with indicated antibodies. The positions of LC3-I and LC3-II are indicated. (C) MCF7 cells transfected and treated as in (B) except for the addition of E64d and pepstatin A at 16 h before harvest. Cell lysates were analysed by western blot with antibodies as indicated. (D) Models for the DAPK degradation pathway mediated by KLHL20–Cul3–ROC1 E3 ligase complex and for the inhibition of this pathway in IFN-treated cells.
Similar articles
- KLHL39 suppresses colon cancer metastasis by blocking KLHL20-mediated PML and DAPK ubiquitination.
Chen HY, Hu JY, Chen TH, Lin YC, Liu X, Lin MY, Lang YD, Yen Y, Chen RH. Chen HY, et al. Oncogene. 2015 Oct 1;34(40):5141-51. doi: 10.1038/onc.2014.435. Epub 2015 Jan 26. Oncogene. 2015. PMID: 25619834 - Cul3-KLHL20 Ubiquitin Ligase Governs the Turnover of ULK1 and VPS34 Complexes to Control Autophagy Termination.
Liu CC, Lin YC, Chen YH, Chen CM, Pang LY, Chen HA, Wu PR, Lin MY, Jiang ST, Tsai TF, Chen RH. Liu CC, et al. Mol Cell. 2016 Jan 7;61(1):84-97. doi: 10.1016/j.molcel.2015.11.001. Epub 2015 Dec 10. Mol Cell. 2016. PMID: 26687681 - BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase.
Furukawa M, Xiong Y. Furukawa M, et al. Mol Cell Biol. 2005 Jan;25(1):162-71. doi: 10.1128/MCB.25.1.162-171.2005. Mol Cell Biol. 2005. PMID: 15601839 Free PMC article. - KLHL20 and its role in cell homeostasis: A new perspective and therapeutic potential.
Ramagoma RB, Makgoo L, Mbita Z. Ramagoma RB, et al. Life Sci. 2024 Nov 15;357:123041. doi: 10.1016/j.lfs.2024.123041. Epub 2024 Sep 3. Life Sci. 2024. PMID: 39233199 Review. - Cullin 3 and Its Role in Tumorigenesis.
Chen RH. Chen RH. Adv Exp Med Biol. 2020;1217:187-210. doi: 10.1007/978-981-15-1025-0_12. Adv Exp Med Biol. 2020. PMID: 31898229 Review.
Cited by
- Death Associated Protein Kinase 1 (DAPK1): A Regulator of Apoptosis and Autophagy.
Singh P, Ravanan P, Talwar P. Singh P, et al. Front Mol Neurosci. 2016 Jun 23;9:46. doi: 10.3389/fnmol.2016.00046. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27445685 Free PMC article. Review. - Cul3-KLHL20 ubiquitin ligase: physiological functions, stress responses, and disease implications.
Chen HY, Liu CC, Chen RH. Chen HY, et al. Cell Div. 2016 Apr 1;11:5. doi: 10.1186/s13008-016-0017-2. eCollection 2016. Cell Div. 2016. PMID: 27042198 Free PMC article. Review. - Downregulation of autophagy through CUL3-KLHL20-mediated turnover of the ULK1 and PIK3C3/VPS34 complexes.
Feng Y, Klionsky DJ. Feng Y, et al. Autophagy. 2016 Jul 2;12(7):1071-2. doi: 10.1080/15548627.2016.1173802. Epub 2016 Apr 20. Autophagy. 2016. PMID: 27096860 Free PMC article. - The emerging family of CULLIN3-RING ubiquitin ligases (CRL3s): cellular functions and disease implications.
Genschik P, Sumara I, Lechner E. Genschik P, et al. EMBO J. 2013 Aug 28;32(17):2307-20. doi: 10.1038/emboj.2013.173. Epub 2013 Aug 2. EMBO J. 2013. PMID: 23912815 Free PMC article. Review. - Degradation of DRAK1 by CUL3/SPOP E3 Ubiquitin ligase promotes tumor growth of paclitaxel-resistant cervical cancer cells.
Pang K, Lee J, Kim J, Park J, Park Y, Hong E, An H, Ooshima A, Son M, Park KS, Cho JH, Lee C, Song YS, Yang KM, Kim SJ. Pang K, et al. Cell Death Dis. 2022 Feb 22;13(2):169. doi: 10.1038/s41419-022-04619-w. Cell Death Dis. 2022. PMID: 35194034 Free PMC article.
References
- Bialik S, Bresnick AR, Kimchi A (2004) DAP-kinase-mediated morphological changes are localization dependent and involve myosin-II phosphorylation. Cell Death Differ 11: 631–644 - PubMed
- Bialik S, Kimchi A (2004) DAP-kinase as a target for drug design in cancer and diseases associated with accelerated cell death. Semin Cancer Biol 14: 283–294 - PubMed
- Cardozo T, Pagano M (2004) The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol 5: 739–751 - PubMed
- Castets M, Coissieux MM, Delloye-Bourgeois C, Bernard L, Delcros JG, Bernet A, Laudet V, Mehlen P (2009) Inhibition of endothelial cell apoptosis by netrin-1 during angiogenesis. Dev Cell 16: 614–620 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases