Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice - PubMed (original) (raw)
Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice
David D Kline et al. J Neurosci. 2010.
Abstract
Postnatal deficits in brain-derived neurotrophic factor (BDNF) are thought to contribute to pathogenesis of Rett syndrome (RTT), a progressive neurodevelopmental disorder caused by mutations in the gene encoding methyl-CpG-binding protein 2 (MeCP2). In Mecp2-null mice, a model of RTT, BDNF deficits are most pronounced in structures important for autonomic and respiratory control, functions that are severely affected in RTT patients. However, relatively little is known about how these deficits affect neuronal function or how they may be linked to specific RTT endophenotypes. To approach these issues, we analyzed synaptic function in the brainstem nucleus tractus solitarius (nTS), the principal site for integration of primary visceral afferent inputs to central autonomic pathways and a region in which we found markedly reduced levels of BDNF in Mecp2 mutants. Our results demonstrate that the amplitude of spontaneous miniature and evoked EPSCs in nTS neurons is significantly increased in Mecp2-null mice and, accordingly, that mutant cells are more likely than wild- type cells to fire action potentials in response to primary afferent stimulation. These changes occur without any increase in intrinsic neuronal excitability and are unaffected by blockade of inhibitory GABA currents. However, this synaptopathy is associated with decreased BDNF availability in the primary afferent pathway and can be rescued by application of exogenous BDNF. On the basis of these findings, we hypothesize that altered sensory gating in nTS contributes to cardiorespiratory instability in RTT and that nTS is a site at which restoration of normal BDNF signaling could help reestablish normal homeostatic controls.
Figures
Figure 1.
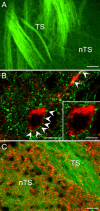
BDNF staining intensity in medullary cell groups is markedly decreased in P35 _Mecp2_-null mice compared to wild-type controls. A1, Representative photomicrograph showing the distribution of tyrosine hydroxylase (TH) protein in the nucleus tractus solitarius region of Mecp2 wild-type mice (Mecp2+/y). A2, A3, BDNF staining in the nucleus tractus solitarius region of Mecp2+/y and null (_Mecp2_−/y) mice, respectively. B1, Cresyl violet staining in the nucleus ambiguus region of Mecp2+/y mice. B2, B3, BDNF staining in the nucleus ambiguus region of Mecp2+/y and null (_Mecp2_−/y) mice, respectively. C1, TH protein staining in A1/C1 region of Mecp2 wild-type mice (Mecp2+/y). C2, C3, BDNF staining in the A1/C1 region of Mecp2+/y and null (_Mecp2_−/y) mice, respectively. A1/C1, A1/C1 catecholaminergic cell group; AP, area postrema; cc, central canal; DMNX, dorsal motor nucleus of the vagus nerve; NA, nucleus ambiguus; RVL, rostroventrolateral reticular nucleus. Scale bar, 200 μm.
Figure 2.
TrkB-positive neurons in the mnTS receive input from BDNF-containing TS fibers. A, Representative picture demonstrating presence of BDNF protein (green) in axons of the TS in the mnTS region of P35 wild-type mice. B, Confocal images of a TrkB-immunoreactive neuron (red) surrounded by BDNF-positive varicosities (green; arrowheads) in the mnTS region. C, DiA-labeled TS axons and varicosities (green) are not immunopositive for TrkB protein (red). Scale bars: 40 μm (A), 10 μm (B), 20 μm (C).
Figure 3.
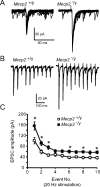
_Mecp2_-null mice exhibit enhanced solitary tract evoked EPSCs. A, B, Representative tracings of TS evoked EPSCs that were recorded from a wild-type (Mecp2+/y, left) and mutant (Mecp2_−/y, right) nTS cell. The TS was stimulated at 0.5 Hz (A, overlay of 20 traces) or 20 Hz (B, average of 5 overlaying traces). C, Average amplitude for each TS-EPSC stimulated at 20 Hz. *p < 0.05, two-way RM ANOVA. Note that TS-EPSC amplitude is higher in Mecp2_−/y mice and frequency-dependent depression is observed.
Figure 4.
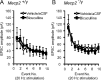
Enhanced EPSCs in _Mecp2_−/y mice are not due to reduced inhibitory shunting. A, Application of 10 μ
m
bicuculline did not alter EPSCs amplitude evoked at 20 Hz in Mecp2+/y mice. Average amplitude for each TS-EPSC stimulated at 20 Hz for aCSF (open diamond) versus bicuculline (closed square). B, Bicuculline (10 μ
m
) did not alter EPSCs amplitude evoked at 20 Hz in _Mecp2_−/y mice. Average amplitude for each TS-EPSC stimulated at 20 Hz for aCSF (open diamond) versus bicuculline (closed square).
Figure 5.
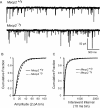
Miniature EPSCs are enhanced in null mice. A, Representative tracings of mEPSCs from a wild-type (top) and null (bottom) mouse nTS cell. Note the increased frequency and amplitude of mEPSCs in null mice. The cell was voltage clamped at −60 mV. B, The cumulative fraction of mEPSC amplitudes (2 pA bin) illustrates a rightward shift in the distribution in null mice. C, The cumulative probability of mEPSC interevent interval distribution (10 ms bin) revealed a significant shift to the left (increased frequency) in null mice.
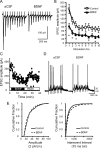
Figure 6.
Exogenous BDNF reduces exaggerated postsynaptic responses in null mice. A, Representative raw trace of evoked TS-EPSCs recorded from a Mecp2_−/y_-null cell during aCSF (left) and following exogenous BDNF (100 ng/ml, 15 min, right). B, Average amplitude for each TS-EPSC stimulated at 20 Hz for aCSF (open square) versus BDNF (closed triangle). *p < 0.05, two-way ANOVA. C, TS-EPSC amplitudes recorded at 0.1 Hz in a null nTS cell in the presence of aCSF, BDNF, and wash out. D, Representative example of TS-evoked discharge in a null nTS cell during aCSF and BDNF (overlap of 5 traces each). Note the decrease in discharge following BDNF. E, In the presence of BDNF, the cumulative fraction of null mEPSC amplitudes (left, 2 pA bin) shifted to the left, while interevent interval distribution (right, 10 ms bin) shifted to the right (decreased frequency).
Similar articles
- Defective GABAergic neurotransmission in the nucleus tractus solitarius in Mecp2-null mice, a model of Rett syndrome.
Chen CY, Di Lucente J, Lin YC, Lien CC, Rogawski MA, Maezawa I, Jin LW. Chen CY, et al. Neurobiol Dis. 2018 Jan;109(Pt A):25-32. doi: 10.1016/j.nbd.2017.09.006. Epub 2017 Sep 18. Neurobiol Dis. 2018. PMID: 28927958 Free PMC article. - A BDNF loop-domain mimetic acutely reverses spontaneous apneas and respiratory abnormalities during behavioral arousal in a mouse model of Rett syndrome.
Kron M, Lang M, Adams IT, Sceniak M, Longo F, Katz DM. Kron M, et al. Dis Model Mech. 2014 Sep;7(9):1047-55. doi: 10.1242/dmm.016030. Dis Model Mech. 2014. PMID: 25147297 Free PMC article. - Excitation/inhibition imbalance and impaired synaptic inhibition in hippocampal area CA3 of Mecp2 knockout mice.
Calfa G, Li W, Rutherford JM, Pozzo-Miller L. Calfa G, et al. Hippocampus. 2015 Feb;25(2):159-68. doi: 10.1002/hipo.22360. Epub 2014 Sep 25. Hippocampus. 2015. PMID: 25209930 Free PMC article. - Exploring the possible link between MeCP2 and oxidative stress in Rett syndrome.
Filosa S, Pecorelli A, D'Esposito M, Valacchi G, Hajek J. Filosa S, et al. Free Radic Biol Med. 2015 Nov;88(Pt A):81-90. doi: 10.1016/j.freeradbiomed.2015.04.019. Epub 2015 May 8. Free Radic Biol Med. 2015. PMID: 25960047 Review. - Brain-derived neurotrophic factor and Rett syndrome.
Katz DM. Katz DM. Handb Exp Pharmacol. 2014;220:481-95. doi: 10.1007/978-3-642-45106-5_18. Handb Exp Pharmacol. 2014. PMID: 24668484 Review.
Cited by
- Anxiety-related mechanisms of respiratory dysfunction in a mouse model of Rett syndrome.
Ren J, Ding X, Funk GD, Greer JJ. Ren J, et al. J Neurosci. 2012 Nov 28;32(48):17230-40. doi: 10.1523/JNEUROSCI.2951-12.2012. J Neurosci. 2012. PMID: 23197715 Free PMC article. - Transcriptional Regulation of Brain-Derived Neurotrophic Factor (BDNF) by Methyl CpG Binding Protein 2 (MeCP2): a Novel Mechanism for Re-Myelination and/or Myelin Repair Involved in the Treatment of Multiple Sclerosis (MS).
KhorshidAhmad T, Acosta C, Cortes C, Lakowski TM, Gangadaran S, Namaka M. KhorshidAhmad T, et al. Mol Neurobiol. 2016 Mar;53(2):1092-1107. doi: 10.1007/s12035-014-9074-1. Epub 2015 Jan 13. Mol Neurobiol. 2016. PMID: 25579386 Review. - Activity-dependent BDNF release and TRPC signaling is impaired in hippocampal neurons of Mecp2 mutant mice.
Li W, Calfa G, Larimore J, Pozzo-Miller L. Li W, et al. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):17087-92. doi: 10.1073/pnas.1205271109. Epub 2012 Oct 1. Proc Natl Acad Sci U S A. 2012. PMID: 23027959 Free PMC article. - Breathing Abnormalities During Sleep and Wakefulness in Rett Syndrome: Clinical Relevance and Paradoxical Relationship With Circulating Pro-oxidant Markers.
Leoncini S, Signorini C, Boasiako L, Scandurra V, Hayek J, Ciccoli L, Rossi M, Canitano R, De Felice C. Leoncini S, et al. Front Neurol. 2022 Mar 29;13:833239. doi: 10.3389/fneur.2022.833239. eCollection 2022. Front Neurol. 2022. PMID: 35422749 Free PMC article. - Global transcriptional and translational repression in human-embryonic-stem-cell-derived Rett syndrome neurons.
Li Y, Wang H, Muffat J, Cheng AW, Orlando DA, Lovén J, Kwok SM, Feldman DA, Bateup HS, Gao Q, Hockemeyer D, Mitalipova M, Lewis CA, Vander Heiden MG, Sur M, Young RA, Jaenisch R. Li Y, et al. Cell Stem Cell. 2013 Oct 3;13(4):446-58. doi: 10.1016/j.stem.2013.09.001. Cell Stem Cell. 2013. PMID: 24094325 Free PMC article.
References
- Andresen MC, Kunze DL. Nucleus tractus solitarius—gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. - PubMed
- Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS) J Neurophysiol. 2008;99:1712–1722. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS057398/NS/NINDS NIH HHS/United States
- R01 NS057398-01/NS/NINDS NIH HHS/United States
- R01 NS057398-02/NS/NINDS NIH HHS/United States
- R01 NS057398-03/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases