3.3 A cryo-EM structure of a nonenveloped virus reveals a priming mechanism for cell entry - PubMed (original) (raw)
3.3 A cryo-EM structure of a nonenveloped virus reveals a priming mechanism for cell entry
Xing Zhang et al. Cell. 2010.
Abstract
To achieve cell entry, many nonenveloped viruses must transform from a dormant to a primed state. In contrast to the membrane fusion mechanism of enveloped viruses (e.g., influenza virus), this membrane penetration mechanism is poorly understood. Here, using single-particle cryo-electron microscopy, we report a 3.3 A structure of the primed, infectious subvirion particle of aquareovirus. The density map reveals side-chain densities of all types of amino acids (except glycine), enabling construction of a full-atom model of the viral particle. Our structure and biochemical results show that priming involves autocleavage of the membrane penetration protein and suggest that Lys84 and Glu76 may facilitate this autocleavage in a nucleophilic attack. We observe a myristoyl group, covalently linked to the N terminus of the penetration protein and embedded in a hydrophobic pocket. These results suggest a well-orchestrated process of nonenveloped virus entry involving autocleavage of the penetration protein prior to exposure of its membrane-insertion finger.
2010 Elsevier Inc. All rights reserved.
Figures
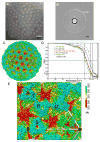
Figure 1. Cryo-EM and 3D Reconstruction of Infectious Subvirion Particle of Aquareovirus
(A) Representative cryo-EM image of the ISVP at 1.2 μm underdefocus. There is no carbon support film, and viral particles are suspended in vitreous ice. (B) Fourier-transformed spectrum of the image in (A), showing contrast transfer function rings visible beyond 1/4 Å-1 and a strong signal at ~1/3.6 Å-1 (dashed arc), which is likely due to H2O (Dowell and Rinfret, 1960). (C) Density map of the aquareovirus ISVP at 3.3 Å resolution, colored according to radius. An enlarged view of an asymmetric unit is shown in Figure 1E. See also Movie S1. (D) Fourier shell cross-correlation coefficients (FSC), indicating the effective resolution to be 3.3 Å for VP3, VP6 and the averaged VP5, and 3.5 Å for VP1 according to the reference-based criterion as defined by Rosenthal and Henderson (Cref=0.5 or FSC=0.143) (Rosenthal and Henderson, 2003). Different curves represent different protein shells: the whole protein shell (red), the shell containing VP1 (black), the shell of VP3 and VP6 (blue) and the VP5 layer (green: the solid line for the original VP5 layer and the dashed line for the averaged VP5). (E) Enlarged view of the cryo-EM density of the primed ISVP, showing an asymmetric unit in Figure1C. Four pseudo equivalent VP5 trimers are labeled (Q, R, S and T), and positions of 2- (ellipse), 3- (triangle) and 5-fold (pentagon) axes are indicated. See also Figure S1 and Movie S1.
Figure 2. Atomic Model of the Aquareovirus ISVP
(A) Cryo-EM density (cyan mesh) of a VP5 monomer superimposed on its atomic model (yellow; only backbone shown). (B) Stereo view of the box from (A). Cryo-EM densities (gray mesh) are superimposed on its atomic model (magenta). (C) Cryo-EM density (cyan mesh) of VP3A superimposed on its atomic model (yellow; only backbone shown). (D) Cryo-EM densities (gray mesh) of the four boxed regions in (C) showing representative side chains. Cryo-EM densities (gray mesh) are superimposed on its atomic model (magenta). Box 1 also shows density for a carboxyl oxygen atom (arrow). The different aromatic amino acids, Trp, Tyr and Phe, in boxes 2-4 are readily distinguished. (E) Complete atomic model of the ISVP. In the right half, removal of the VP5 coat reveals core proteins. Ribbon models of the atomic structures of the six conformers from four structural proteins are shown in the periphery: two VP3 conformers (Figure S1), two VP6 conformers (Figure S2 and Movie S2) one VP1 (Figure S3 and Movie S2), and one VP5. These atomic models are color-coded according to amino acid sequence from blue (N terminus) through green and yellow to red (C-terminus). The black triangle demarcates a VP5 trimer. See also Figure S2 and Movie S2.
Figure 3. Structural Comparison of Primed and Dormant Penetration Proteins
Atomic model of a primed VP5 trimer (magenta ribbon) is superimposed on the atomic model of a dormant μ1 trimer of orthoreovirus (green ribbon), showing different conformations for some of the loops in the helix-rich region but similar conformations for the remainder of the helix-rich region. The jelly-roll domain, indicated by a dashed box.
Figure 4. Cleavage Sites of the Membrane Penetration Protein VP5
(A) VP5 trimer reviewed from side (inset; see also Movie S4) and from inside of ISVP (indicated by an arrow in the inset). Each monomer of the trimer contains an autocleavage site at Asn42-Pro43. The Cα atoms of Asn42 (red ball) and Pro43 (green ball) are indicated. Only a 30 Å thick slab (indicated by the two horizontal lines in the inset) at the trimer base of the trimer is shown for clarity. (B) A enlarged view of the boxed region of one VP5 monomer in (A), except the cryo-EM densities are also shown (gray mesh). Autocleavage of the Asn42-Pro43 bond is indicated by an 11.7Å gap between their Cα atoms. (C) Side view of the ribbon model of a VP5 trimer and the position of the intact δ-ϕ proteolytic cleavage loop (residues: 550-561) (dashed box). Cryo-EM density (gray mesh) of the boxed region of one VP5 monomer is also shown, demonstrating an uncleaved loop from residues 551 to 560. (D) Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of aquareovirus virion (dormant) and ISVP (primed) from different bands of the same CsCl gradient, showing intact VP5 in virions and autocleaved VP5 (i.e., VP5C, bands indicated by asterisks) in ISVPs. Lane (M), molecular weight markers. (E) Lys84 (cyan ball) near Asn42 (red ball) at the autocleavage site, and an adjacent Glu76 (green ball). See also Movie S3.
Figure 5. Myristoyl Group of the Primed VP5 in the ISVP
(A) Positions of three myristoyl groups (red balls) near the base of the VP5 trimer. See also Movie S4. (B) Cryo-EM density (gray mesh) of the base region in Figure 5A (red box), superimposed with its atomic model (magenta), showing the myristoyl group (red balls and sticks) inside a hydrophobic pocket. See also Movie S4. (C) Amide linkage of the myristoyl group to Gly2. (D) Changes of the penetration protein VP5 from a stable, dormant state to a metastable, primed ISVP state and subsequently to the ISVP* state. Red, base domain (2-242); green, linker domain (243-286 & 485-648) and cyan, jelly-roll domain (287-484). The myristoyl group (myr) in the hydrophobic pocket is enclosed by an ellipse; the released myristoyl group has no ellipse.
Figure 6. Infection and Replication of Aquareovirus at 4°C
(A) Mock infected CIK. (B) and (C) aquareovirus infected CIK cell at 4°C and 3 days post infection. Arrows indicate the cytopathic effects (CPE) in infected cells. (D) Immunoblot analysis of viral NS80 expression. Lane (M), Molecular weight markers; Lane 1, Mock infected CIK cell lysate showing the NS80 protein expression level of 0% as negative control (measured by relative optical density: 0%, no absorbance; 100%, complete absorbance); Lanes 2 and 3, aquareovirus infected CIK cell lysate at 4°C showing the NS80 protein expression levels of 6.6% and 5.8% at 5 days post infection; Lane 4, aquareovirus infected CIK cell lysate at 28°C showing the NS80 protein expression level of 58.7% at 2 days post infection; Lane 5, E. coli expressed NS80 at level of 56% as positive control. Experiments were performed in triplets, and the mean optical density of the triplet immunoblots were shown (error bars represent standard deviation).
Similar articles
- In Situ Structures of the Polymerase Complex and RNA Genome Show How Aquareovirus Transcription Machineries Respond to Uncoating.
Ding K, Nguyen L, Zhou ZH. Ding K, et al. J Virol. 2018 Oct 12;92(21):e00774-18. doi: 10.1128/JVI.00774-18. Print 2018 Nov 1. J Virol. 2018. PMID: 30068643 Free PMC article. - Primed for Discovery: Atomic-Resolution Cryo-EM Structure of a Reovirus Entry Intermediate.
Trask SD, Guglielmi KM, Patton JT. Trask SD, et al. Viruses. 2010 Jun;2(6):1340-1346. doi: 10.3390/v2061340. Epub 2010 Jun 15. Viruses. 2010. PMID: 21994683 Free PMC article. - Electron microscopic imaging revealed the flexible filamentous structure of the cell attachment protein P2 of Rice dwarf virus located around the icosahedral 5-fold axes.
Miyazaki N, Higashiura A, Higashiura T, Akita F, Hibino H, Omura T, Nakagawa A, Iwasaki K. Miyazaki N, et al. J Biochem. 2016 Feb;159(2):181-90. doi: 10.1093/jb/mvv092. Epub 2015 Sep 15. J Biochem. 2016. PMID: 26374901 Free PMC article. - High-resolution 3D structures reveal the biological functions of reoviruses.
Li X, Fang Q. Li X, et al. Virol Sin. 2013 Dec;28(6):318-25. doi: 10.1007/s12250-013-3341-6. Epub 2013 Nov 6. Virol Sin. 2013. PMID: 24254888 Free PMC article. Review. - Breach: Host Membrane Penetration and Entry by Nonenveloped Viruses.
Kumar CS, Dey D, Ghosh S, Banerjee M. Kumar CS, et al. Trends Microbiol. 2018 Jun;26(6):525-537. doi: 10.1016/j.tim.2017.09.010. Epub 2017 Oct 25. Trends Microbiol. 2018. PMID: 29079499 Review.
Cited by
- Grass carp reovirus NS26 interacts with cellular lipopolysaccharide-induced tumor necrosis factor-alpha factor, LITAF.
Lu J, Wang H, Zhang Y, Li Y, Lu L. Lu J, et al. Virus Genes. 2016 Dec;52(6):789-796. doi: 10.1007/s11262-016-1370-6. Epub 2016 Jul 12. Virus Genes. 2016. PMID: 27405988 - Outcome of the first electron microscopy validation task force meeting.
Henderson R, Sali A, Baker ML, Carragher B, Devkota B, Downing KH, Egelman EH, Feng Z, Frank J, Grigorieff N, Jiang W, Ludtke SJ, Medalia O, Penczek PA, Rosenthal PB, Rossmann MG, Schmid MF, Schröder GF, Steven AC, Stokes DL, Westbrook JD, Wriggers W, Yang H, Young J, Berman HM, Chiu W, Kleywegt GJ, Lawson CL. Henderson R, et al. Structure. 2012 Feb 8;20(2):205-14. doi: 10.1016/j.str.2011.12.014. Structure. 2012. PMID: 22325770 Free PMC article. - Low cost, high performance GPU computing solution for atomic resolution cryoEM single-particle reconstruction.
Zhang X, Zhang X, Zhou ZH. Zhang X, et al. J Struct Biol. 2010 Dec;172(3):400-6. doi: 10.1016/j.jsb.2010.05.006. Epub 2010 May 21. J Struct Biol. 2010. PMID: 20493949 Free PMC article. - A cool hybrid approach to the herpesvirus 'life' cycle.
Zeev-Ben-Mordehai T, Hagen C, Grünewald K. Zeev-Ben-Mordehai T, et al. Curr Opin Virol. 2014 Apr;5(100):42-9. doi: 10.1016/j.coviro.2014.01.008. Epub 2014 Feb 16. Curr Opin Virol. 2014. PMID: 24553093 Free PMC article. Review. - Building a pseudo-atomic model of the anaphase-promoting complex.
Kulkarni K, Zhang Z, Chang L, Yang J, da Fonseca PC, Barford D. Kulkarni K, et al. Acta Crystallogr D Biol Crystallogr. 2013 Nov;69(Pt 11):2236-43. doi: 10.1107/S0907444913018593. Epub 2013 Oct 12. Acta Crystallogr D Biol Crystallogr. 2013. PMID: 24189235 Free PMC article.
References
- Banerjee M, Johnson JE. Activation, exposure and penetration of virally encoded, membrane-active polypeptides during non-enveloped virus entry. Curr Protein Pept Sci. 2008;9:16–27. - PubMed
- Bartlett GJ, Porter CT, Borkakoti N, Thornton JM. Analysis of catalytic residues in enzyme active sites. J Mol Biol. 2002;324:105–121. - PubMed
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1S10RR23057/RR/NCRR NIH HHS/United States
- R01 AI069015/AI/NIAID NIH HHS/United States
- GM071940/GM/NIGMS NIH HHS/United States
- R01 GM071940/GM/NIGMS NIH HHS/United States
- AI069015/AI/NIAID NIH HHS/United States
- S10 RR023057/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources