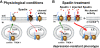Spadin, a sortilin-derived peptide, targeting rodent TREK-1 channels: a new concept in the antidepressant drug design - PubMed (original) (raw)
. 2010 Apr 13;8(4):e1000355.
doi: 10.1371/journal.pbio.1000355.
Olivier Pétrault, Guillaume Lucas, Emmanuel Deval, Sophie Béraud-Dufour, Carine Gandin, Malika El-Yacoubi, Catherine Widmann, Alice Guyon, Eric Chevet, Said Taouji, Grégory Conductier, Alain Corinus, Thierry Coppola, Gabriella Gobbi, Jean-Louis Nahon, Catherine Heurteaux, Marc Borsotto
Affiliations
- PMID: 20405001
- PMCID: PMC2854129
- DOI: 10.1371/journal.pbio.1000355
Spadin, a sortilin-derived peptide, targeting rodent TREK-1 channels: a new concept in the antidepressant drug design
Jean Mazella et al. PLoS Biol. 2010.
Abstract
Current antidepressant treatments are inadequate for many individuals, and when they are effective, they require several weeks of administration before a therapeutic effect can be observed. Improving the treatment of depression is challenging. Recently, the two-pore domain potassium channel TREK-1 has been identified as a new target in depression, and its antagonists might become effective antidepressants. In mice, deletion of the TREK-1 gene results in a depression-resistant phenotype that mimics antidepressant treatments. Here, we validate in mice the antidepressant effects of spadin, a secreted peptide derived from the propeptide generated by the maturation of the neurotensin receptor 3 (NTSR3/Sortilin) and acting through TREK-1 inhibition. NTSR3/Sortilin interacted with the TREK-1 channel, as shown by immunoprecipitation of TREK-1 and NTSR3/Sortilin from COS-7 cells and cortical neurons co-expressing both proteins. TREK-1 and NTSR3/Sortilin were colocalized in mouse cortical neurons. Spadin bound specifically to TREK-1 with an affinity of 10 nM. Electrophysiological studies showed that spadin efficiently blocked the TREK-1 activity in COS-7 cells, cultured hippocampal pyramidal neurons, and CA3 hippocampal neurons in brain slices. Spadin also induced in vivo an increase of the 5-HT neuron firing rate in the Dorsal Raphe Nucleus. In five behavioral tests predicting an antidepressant response, spadin-treated mice showed a resistance to depression as found in TREK-1 deficient mice. More importantly, an intravenous 4-d treatment with spadin not only induced a strong antidepressant effect but also enhanced hippocampal phosphorylation of CREB protein and neurogenesis, considered to be key markers of antidepressant action after chronic treatment with selective serotonin reuptake inhibitors. This work also shows the development of a reliable method for dosing the propeptide in serum of mice by using AlphaScreen technology. These findings point out spadin as a putative antidepressant of new generation with a rapid onset of action. Spadin can be regarded as the first natural antidepressant peptide identified. It corresponds to a new concept to address the treatment of depression.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
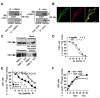
Figure 1. NTSR3/Sortilin and Spadin interact with the TREK-1 channel.
(A) Immunoprecipitation of NTSR3/Sortilin with anti-TREK-1 antibodies (IP α-TREK-1) or of TREK-1 with anti-NTSR3/Sortilin antibodies (IP α-Sort) from transfected COS-7 cells or mouse cortical neurons. Immunoprecipitated proteins were subjected to Western blots and revealed using anti-sortilin (WB: α-Sort) or anti-TREK-1 (WB: α-TREK-1). (B) Double immunofluorescence labeling of TREK-1 (Green) and NTSR3/Sortilin (Red) in mouse cortical neurons. Nuclei were labeled using Dapi (Blue) and co-localized proteins were visualized using merge images (arrows); scale bar, 10 µm. (C) Influence of NTSR3/Sortilin on the expression of TREK-1 at the plasma membranes. COS-7 cells were transfected with TREK-1 in the absence or in the presence of NTSR3/Sortilin. Crude homogenates, purified plasma membrane proteins, or cell surface biotinylated proteins were subjected to Western blot analysis and revealed using anti-TREK-1 antibodies. (D) Competition between 125I-NT and unlabeled Spadin (closed circles) or NT (open circles) for binding to C13NJ cell homogenates. Each point represents the mean of duplicate determinations from 3 independent experiments. (E) Competition between 125I-Spadin and unlabeled Spadin (closed circles), NT (open circles) or N-terminal fragment Gln1-Arg 16 (Nterm1-16, open triangles) for binding to TREK-1 transfected COS-7 cell homogenates. Each point represents the mean of duplicate determinations from 2 to 5 independent experiments. Note that non-transfected COS-7 cells were totally devoid of 125I-Spadin binding. (F) Association kinetics of 125I-Spadin binding to COS-7 cells transfected with TREK-1. At the indicated times, cells were either washed twice with 500 µl of binding buffer (closed circles) or treated with 500 µl of acid-NaCl buffer for 2 min (open circles).
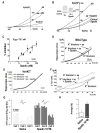
Figure 2. AlphaScreen assays.
(A) Principles of AlphaScreen technology. Donor and acceptor microbeads can be coated with target-specific antibody, proteins, or secondary reagents (streptavidin, glutathione, nickel). A signal is produced when the AlphaScreen acceptor, A, and donor, D, beads are brought into proximity by a molecular interaction occurring between the binding partners captured on the beads. Laser excitation at 680 nm causes ambient oxygen to be converted to the singlet state by photosynthesizers on the donor bead. These react with chemiluminescent agents on the Acceptor bead only when the latter is in close proximity, emitting light at 520–620 nm. Here, we illustrate a competition protocol between seric propeptide, PE, and interacting donor beads, D, coupled-biotinylated spadin (b-spadin) with antibodies anti-propeptide (anti-PE) coupled on acceptor beads, A. (B) An example of competition curve obtained with one group (n° 1) of 6 mice (1.1 to 1.6) among 5 different groups (other curves are presented in the Figure S1). Values obtained are compared to the standard curve. (C) Seric concentrations of the full length propeptide calculated for the 5 groups from competition experiments as shown in (B).
Figure 3. Effects of Spadin on the TREK-1 channel activity.
(A–B) Whole-cell currents measured in COS-7 transfected cells in presence of potassium blockers (K+ blockers, 10 mM tetraethyl ammonium (TEA), 3 mM 4-aminopyridine (4-AP), 50 nM charybdotoxin, 10 µM glibenclamide, 100 nM apamin). Cells were clamped at −80 mV and voltage changes were either applied by ramp from −100 to 50 mV, 1 s in duration (A, B main panel) or by 10mV steps from −100 to 40 mV, 1.5 s in duration (B inset). Currents were recorded after TREK-1 activation by 10 µM arachidonic acid (aa) and aa + propeptide (PE, 500 nM) (A) or aa + Spadin (100 nM) (B). Native currents were recorded in absence (Control) and in presence of spadin (Spadin 100 nM, B). Peptides were applied via the bath medium. (C) Dose-dependent spadin inhibition of TREK-1 currents, IC50 value at 0 mV is of 70.7 nM. Currents were measured in presence of 10 µM aa. (D–E) Native currents recorded in the presence of K+ blockers after stimulation by 10 µM of aa on CA3 pyramidal neurons from hippocampus slices in wild-type mice (D) or in kcnk2 deficient mice (kcnk2 −/−) (E) in the presence or the absence of spadin (1 µM). Currents were elicited by a ramp from −100 mV to 50 mV. (F) Native currents on β-TC3 cell line in similar experimental conditions as (D–E). (G–H) Effect of spadin on the firing rate of DRN 5-HT neurons. Spadin (10−5 M in a 100 µl bolus) or its vehicle was i.p. administered. Recordings started 30 min after the injection and were performed for a maximal duration of 210 min thereafter. (G) Main panel: Samples of “descents” performed along the DRN, showing typical integrated firing rate histograms in a vehicle- (left panel) or in a spadin-treated (right panel) animal. Each cluster represents the electrical activity of one neuron, each bar representing the average number of recorded action potentials per 10 s. Insets, examples of action potential waveforms of 5-HT neurons. (H) 5-HT neuron firing activity, calculated on the basis of all the cells recorded within the successive tracks performed along the DRN. Values at the bottom of each column indicate the total number of neurons recorded (n = 4 mice in both groups).
Figure 4. Acute antidepressant effects of Spadin.
(A–E) Acute treatments: Spadin (10−4 to 10−8 M) or Fluoxetine (3 mg/kg) or Saline solutions were injected 30 min before the test in wild-type and kcnk2 −/− mice (A, B, C). (A) Forced Swimming Test (FST, n = 10 per group), spadin-treated mice had a shorter time of immobility comparable to those obtained with kcnk2 −/− or fluoxetine-treated mice, whatever the way of spadin administration: intracerebroventricular (i.c.v., n = 14 per group) (one-way ANOVA, F 3,55 = 79.53, ***p<0.001 versus saline-treated mice), intravenous (i.v., n = 8 per group except for fluoxetine and kcnk2 −/− groups, n = 6) (one-way ANOVA, F 5,43 = 26.27, ***p<0.001 versus saline-treated mice) or intraperitoneal (i.p., n = 10 per group except for kcnk2 −/−, n = 5 ) (one-way ANOVA, F 3,34 = 40.58, *p<0.05, ***p<0.001 versus saline-treated mice). (B) Tail Suspension Test (TST, n = 15 for saline and spadin groups, and n = 9 for fluoxetine and kcnk2 −/− groups), i.v. spadin-treated mice had a shorter immobility score comparable to those obtained with kcnk2 −/− or fluoxetine-treated mice (one-way ANOVA, F 3,47 = 11.40, **p<0.01, ***p<0.001 versus saline-treated mice). (C) Conditioned Motility Suppression Test (CMST, n = 10 per group). Two-way ANOVA showed significant effects of shocks (F 1,62 = 254.1, p<0.001), treatment (F 3,62 = 3.87, p<0.01) and an interaction between these two factors (F 3,62 = 8.83, p<0.001). ### p<0.01 versus non-shocked mice. In the shocked groups, spadin treatment reversed the freezing state induced by the shock training in saline-treated mice (78±7 versus 14±2 counts, respectively). This effect was stronger than those observed for kcnk2 −/− or fluoxetine-treated mice (one-way ANOVA, F 3,39 = 10,87, *p<0.05, ***p<0.001 versus saline-treated mice). Counts are the number of squares crossed plus the number of climbings. (D and E) Learned Helplessness test (LH, n = 12 per group). Shocked spadin-treated mice showed shorter escape latencies than saline-treated mice. Two-way ANOVA showed significant effect for treatment (F1,110 = 7.93, p = 0.01) and for assay (F5,110 = 3.56, p = 0.005 , *p<0.05 in shocked groups). (D) Mean escape latencies ± SEM averaged in 6 blocks of 5 trials, and (E) mean overall latency ± SEM to escape across trials 1–30 as a function of spadin treatment. Two-way ANOVA (Shocks×Treatment) showed an interaction between these two factors (F1,44 = 6.9, p = 0.012). ## p = 0.007 for non-shocked saline-treated mice versus shocked saline-treated mice.
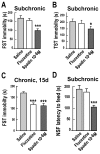
Figure 5. Subchronic and chronic antidepressant effects of Spadin.
Subchronic treatments: Spadin (10−6 M), Fluoxetine (3 mg/kg), or Saline solutions were i.v. injected in a 100 µL bolus once a day for 4 successive d before the test. In chronic treatments, spadin (10−6 M) and fluoxetine (1 mg/kg) were i.v. injected in a 100 µL bolus once a day for 15 successive d. For each test there were 8 animals per group. (A) In FST (one-way ANOVA, F 2,23 = 26.08, ***p<0.001) and (B) TST (one-way ANOVA, F 2,24 = 9.8, *p<0.05 versus saline-treated mice), spadin induced similar behaviors than those obtained with the acute treatment, whereas fluoxetine was without effect . (C) In FST, chronic treatment with spadin or fluoxetine significantly reduced the time of immobility (one-way ANOVA, F2,26 = 25.08, ***p<0.001 versus saline-treated mice). (D) NSF paradigm: at the end of the 4 d treatment, animals were food deprived for 1 d and then measured for their latency to feed. Spadin treatment significantly reduced the latency to feed when compared to saline or fluoxetine treatments (t test, ***p<0.001 versus saline-treated mice). In all graphs, data are expressed as means ± SEM.
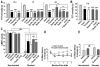
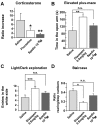
Figure 6. Effect of Spadin on stress and anxiety behaviors.
(A) Decreased stress-induced serum levels of corticosterone in mice treated with spadin. We compared serum corticosterone concentrations (ng/ml) sampled in the morning in mice acutely treated with spadin (i.v, 10−6 M), saline or fluoxetine (i.p., 3 mg/kg) 30 min after a 10 min tube restraint (n = 10 per group). Data are expressed as increase of the ratio corticosterone levels 30 min after stress over basal corticosterone levels 30 min before restraint (one-way ANOVA, F2,27 = 18.30, *p<0.05, **p<0.01 versus saline-treated mice). (B) Effect of spadin (i.p, 10−5 M) and diazepam (i.p., 0.5 mg/kg) on time spent in the open arms (s) of the elevated plus-maze (n = 10 per group, one-way ANOVA, F2,27 = 8.75, **p<0.001 versus saline-treated mice). (C) Effect of spadin (i.p., 10−5 M) and diazepam (i.p., 0.5 mg/kg) on the total number of entries in the aversive white side in the light/dark transition test (n = 10 per group, one-way ANOVA, F2,53 = 7.65, ***p = 0.001 versus saline-treated mice). (D) Influence of spadin (i.p., 10−5 M) and diazepam (i.p., 0.5 mg/kg) on mouse performance in the staircase test. Data are presented as the ratio of number of rearings over the number of ascended steps (n = 10 per group, one-way ANOVA, F2,44 = 4.86, *p<0.05 versus saline-treated mice). In the three tests, mice were injected with either spadin or diazepam 30 min before the test. In all graphs, bars indicate SEM.
Figure 7. Effects of Spadin on neurogenesis and CREB activation.
(A–B) Spadin increased neurogenesis (A). Top, representative photomicrographs of BrdU-labeled neurons in the dentate gyrus of the mouse hippocampus treated either with saline or with spadin (i.v., 10−6 M) for 4 d. Bottom, double labeling of BrdU-labeled neurons either with GFAP (glial marker) or with DCX (neuronal precursor marker), showing a co-localization only with DCX, and not with GFAP. (B) Quantitation of BrdU positive cells of hippocampus treated with saline, fluoxetine, or spadin (10−5 M) for 4 d. 85% of BrdU-labeled cells were positive to DCX. Data are number of BrdU+ or DCX+ cells in mouse hippocampus (n = 5) (F2,53 = 35.27; ***p<0.001 versus saline). (C) Quantitation of BrdU positive cells of hippocampus treated with saline, fluoxetine, or spadin (10−5 M) for 15 d (n = 5) (F2,53 = 19.43; *p<0.05, **p<0.01 versus saline). (D–G) Enhanced spadin treatment-induced CREB activation in the hippocampus, as assessed by measuring phosphoCREB (pCREB) immunoreactivity. (D) Immunological distribution of pCREB in the mouse hippocampus after a 4 d i.v. treatment. pCREB is phosphorylated in the cells near the subgranular zone (SGZ). (E) Quantification of pCREB positive cells/mm2 in hippocampal SGZ (n = 5) (t test; ***p<0.001). (F) Western blot analysis of pCREB level in hippocampus treated with saline or spadin (10−5 M). (G) Double immunofluorescent staining (examples are indicated by arrows) for pCREB and DCX positive hippocampal neurons treated with saline or spadin (10−5 M).
Figure 8. Schematic model of TREK-1 regulation by NTSR3/Sortilin and Spadin.
In physiological conditions (A) the concentration of spadin that would be released from vesicles of the Trans Golgi Network (TGN) is not sufficient to completely abolish the channel activity, by internalization via Early Endosome (E.E.) vesicles, direct blockade, or both. Conversely, under spadin treatment (B) the amount of spadin is sufficient to internalize all channel molecules and consequently to abolish the channel activity.
Similar articles
- Spadin as a new antidepressant: absence of TREK-1-related side effects.
Moha Ou Maati H, Veyssiere J, Labbal F, Coppola T, Gandin C, Widmann C, Mazella J, Heurteaux C, Borsotto M. Moha Ou Maati H, et al. Neuropharmacology. 2012 Jan;62(1):278-88. doi: 10.1016/j.neuropharm.2011.07.019. Epub 2011 Jul 22. Neuropharmacology. 2012. PMID: 21807005 - Retroinverso analogs of spadin display increased antidepressant effects.
Veyssiere J, Moha Ou Maati H, Mazella J, Gaudriault G, Moreno S, Heurteaux C, Borsotto M. Veyssiere J, et al. Psychopharmacology (Berl). 2015 Feb;232(3):561-74. doi: 10.1007/s00213-014-3683-2. Epub 2014 Aug 2. Psychopharmacology (Berl). 2015. PMID: 25080852 Free PMC article. - First evidence of protective effects on stroke recovery and post-stroke depression induced by sortilin-derived peptides.
Pietri M, Djillani A, Mazella J, Borsotto M, Heurteaux C. Pietri M, et al. Neuropharmacology. 2019 Nov 1;158:107715. doi: 10.1016/j.neuropharm.2019.107715. Epub 2019 Jul 17. Neuropharmacology. 2019. PMID: 31325429 - Targeting two-pore domain K(+) channels TREK-1 and TASK-3 for the treatment of depression: a new therapeutic concept.
Borsotto M, Veyssiere J, Moha Ou Maati H, Devader C, Mazella J, Heurteaux C. Borsotto M, et al. Br J Pharmacol. 2015 Feb;172(3):771-84. doi: 10.1111/bph.12953. Epub 2014 Nov 24. Br J Pharmacol. 2015. PMID: 25263033 Free PMC article. Review. - Fighting against depression with TREK-1 blockers: Past and future. A focus on spadin.
Djillani A, Pietri M, Mazella J, Heurteaux C, Borsotto M. Djillani A, et al. Pharmacol Ther. 2019 Feb;194:185-198. doi: 10.1016/j.pharmthera.2018.10.003. Epub 2018 Oct 3. Pharmacol Ther. 2019. PMID: 30291907 Review.
Cited by
- Tick-Derived Peptide Blocks Potassium Channel TREK-1.
Du C, Chen L, Liu G, Yuan F, Zhang Z, Rong M, Mo G, Liu C. Du C, et al. Int J Mol Sci. 2024 Jul 31;25(15):8377. doi: 10.3390/ijms25158377. Int J Mol Sci. 2024. PMID: 39125945 Free PMC article. - Osmotically Sensitive TREK Channels in Rat Articular Chondrocytes: Expression and Functional Role.
Ponce A, Ogazon Del Toro A, Jimenez L, Roldan ML, Shoshani L. Ponce A, et al. Int J Mol Sci. 2024 Jul 18;25(14):7848. doi: 10.3390/ijms25147848. Int J Mol Sci. 2024. PMID: 39063089 Free PMC article. - Excitotoxic Storms of Ischemic Stroke: A Non-neuronal Perspective.
Yang XM, Yu H, Li JX, Li N, Li C, Xu DH, Zhang H, Fang TH, Wang SJ, Yan PY, Han BB. Yang XM, et al. Mol Neurobiol. 2024 Nov;61(11):9562-9581. doi: 10.1007/s12035-024-04184-7. Epub 2024 Apr 25. Mol Neurobiol. 2024. PMID: 38662299 Review. - Sortilin-Mediated Inhibition of TREK1/2 Channels in Primary Sensory Neurons Promotes Prediabetic Neuropathic Pain.
Sun W, Yang F, Wang Y, Yang Y, Du R, Wang XL, Luo ZX, Wu JJ, Chen J. Sun W, et al. Adv Sci (Weinh). 2024 Jun;11(23):e2310295. doi: 10.1002/advs.202310295. Epub 2024 Apr 16. Adv Sci (Weinh). 2024. PMID: 38626370 Free PMC article. - Pharmacological inhibition of Kir4.1 evokes rapid-onset antidepressant responses.
Zhou X, Zhao C, Xu H, Xu Y, Zhan L, Wang P, He J, Lu T, Gu Y, Yang Y, Xu C, Chen Y, Liu Y, Zeng Y, Tian F, Chen Q, Xie X, Liu J, Hu H, Li J, Zheng Y, Guo J, Gao Z. Zhou X, et al. Nat Chem Biol. 2024 Jul;20(7):857-866. doi: 10.1038/s41589-024-01555-y. Epub 2024 Feb 14. Nat Chem Biol. 2024. PMID: 38355723
References
- Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thümmler S, et al. Deletion of TREK-1, a background potassium channel, results in a depression-resistant phenotype. Nature Neurosci. 2006;9:1134–1141. - PubMed
- Lesage F, Lazdunski M. Molecular and functional properties of two pore domain potassium channels. Am J Physiol. 2000;279:793–801. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous