Magnetic resonance imaging-guided delivery of adeno-associated virus type 2 to the primate brain for the treatment of lysosomal storage disorders - PubMed (original) (raw)
. 2010 Sep;21(9):1093-103.
doi: 10.1089/hum.2010.040.
A P Kells, R M Richardson, P Hadaczek, J Forsayeth, J Bringas, S P Sardi, M A Passini, L S Shihabuddin, S H Cheng, M S Fiandaca, K S Bankiewicz
Affiliations
- PMID: 20408734
- PMCID: PMC2936496
- DOI: 10.1089/hum.2010.040
Magnetic resonance imaging-guided delivery of adeno-associated virus type 2 to the primate brain for the treatment of lysosomal storage disorders
E Aguilar Salegio et al. Hum Gene Ther. 2010 Sep.
Abstract
Gene replacement therapy for the neurological deficits caused by lysosomal storage disorders, such as in Niemann-Pick disease type A, will require widespread expression of efficacious levels of acid sphingomyelinase (ASM) in the infant human brain. At present there is no treatment available for this devastating pediatric condition. This is partly because of inherent constraints associated with the efficient delivery of therapeutic agents into the CNS of higher order models. In this study we used an adeno-associated virus type 2 (AAV2) vector encoding human acid sphingomyelinase tagged with a viral hemagglutinin epitope (AAV2-hASM-HA) to transduce highly interconnected CNS regions such as the brainstem and thalamus. On the basis of our data showing global cortical expression of a secreted reporter after thalamic delivery in nonhuman primates (NHPs), we set out to investigate whether such widespread expression could be enhanced after brainstem infusion. To maximize delivery of the therapeutic transgene throughout the CNS, we combined a single brainstem infusion with bilateral thalamic infusions in naive NHPs. We found that enzymatic augmentation in brainstem, thalamic, cortical, as well subcortical areas provided convincing evidence that much of the large NHP brain can be transduced with as few as three injection sites.
Figures
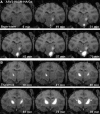
FIG. 1.
Intraoperative use of real-time convection-enhanced delivery (CED) in the nonhuman primate (NHP) brainstem and thalamus. Infusion of AAV2-hASM-HA/Gd visualized as a contrast demarcation on MRI indicates cannula tip placement in the targeted region (A and B; white arrows). Note the increase in infusate size as a function of time as demonstrated in sequential MR image acquisitions. In (A), residual contrast signal visible 19 min after initiation of brainstem infusion correlates with diffusion of thalamic signal, completed 30 min before the end of brainstem infusion.
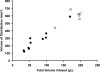
FIG. 2.
Coinfusion of AAV2-hASM-HA and gadoteridol (Gd) into the brainstem (open circles) and thalamus (solid circles). Shown are the results of single delivery of various amounts of infusate into the brainstem (open circles, n = 6; mean _V_i:_V_d ratio, 3.3 ± 0.17) and thalamus (solid circles, n = 10; mean _V_i:_V_d ratio, 3.86 ± 0.25). There is a linear relationship between _V_d and _V_i (overall, n = 16, _R_2 = 0.93), with higher _V_i delivered to the brainstem region as compared with the thalamus. No significant difference was found between ratios in these two regions (p > 0.05).
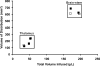
FIG. 3.
Comparison of _V_d:_V_i ratios with repeated brainstem and thalamic infusions. Initial _V_d:_V_i delivery parameters during Gd-only infusions (solid squares, n = 5; mean _V_d:_V_i ratio, 3.74 ± 0.25; _R_2 = 0.96) were replicated in later infusions consisting of AAV2-hASM-HA/Gd (open squares, n = 5; mean _V_d:_V_i ratio, 3.72 ± 0.24; _R_2 = 0.98). Note the consistent distribution patterns in consecutive infusions with or without therapeutic agent (overall, n = 10, _R_2 = 0.96). No significant difference was found between primary and secondary infusions (p > 0.05).
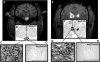
FIG. 4.
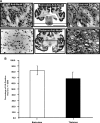
AAV infusion and transduction in brainstem and thalamus. Shown are DICOM MR images representative of brainstem and thalamic infusion (A and E, respectively), and immunostained brain sections anatomically matched to the corresponding MR image (B and F). High-power magnification images demonstrate infusion epicenter with significant neuronal transduction (HA expression) in each targeted region (C and D), as compared with negative controls (G and H). Note that boxes drawn on the MR images are merely representative of the ROIs and are not to scale. Microscopic analysis was performed on NHP tissue perfused 5 weeks after AAV2-hASM-HA infusion. Scale bars: (B and F) 2 cm; (C, D, G, and H) 500 μm.
FIG. 5.
High neuronal transduction numbers in targeted regions. (A) Cellular counting of randomized adjacent, histology-processed sections stained against neuronal marker (anti-NeuN) and HA tag epitope (anti-HA). (B) High neuronal transduction levels in the brainstem (82 ± 7.8%, n = 4) and in the thalamus (68 ± 11.3%, n = 7). No significant difference was found between levels of transduction efficiency in these two regions (p > 0.05). Microscopic analysis was performed on NHP tissue perfused 5 weeks after AAV2-hASM-HA infusion. Scale bars: for whole brain mounts, 2 cm; for immunostained sections, 500 μm.
FIG. 6.
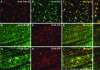
Efficient neuronal transduction in brainstem. Immunostained brainstem sections indicate neuron-specific transduction (A–C) with no transduction observed in astrocytes (D–F) and/or microglia (G–I) (see high-magnification panels on the right). Note that the total number of colocalized NeuN/HA-positive cells was 61 of 63, representing 97% neuronal specificity. Scale bar: (A–I) 500 μm. Color images available online at
.
Similar articles
- Safety study of adeno-associated virus serotype 2-mediated human acid sphingomyelinase expression in the nonhuman primate brain.
Salegio EA, Samaranch L, Jenkins RW, Clarke CJ, Lamarre C, Beyer J, Kells AP, Bringas J, Sebastian WS, Richardson RM, Rosenbluth KH, Hannun YA, Bankiewicz KS, Forsayeth J. Salegio EA, et al. Hum Gene Ther. 2012 Aug;23(8):891-902. doi: 10.1089/hum.2012.052. Hum Gene Ther. 2012. PMID: 22574943 Free PMC article. - Adeno-associated viral vector serotype 9-based gene therapy for Niemann-Pick disease type A.
Samaranch L, Pérez-Cañamás A, Soto-Huelin B, Sudhakar V, Jurado-Arjona J, Hadaczek P, Ávila J, Bringas JR, Casas J, Chen H, He X, Schuchman EH, Cheng SH, Forsayeth J, Bankiewicz KS, Ledesma MD. Samaranch L, et al. Sci Transl Med. 2019 Aug 21;11(506):eaat3738. doi: 10.1126/scitranslmed.aat3738. Sci Transl Med. 2019. PMID: 31434754 Free PMC article. - Merits of combination cortical, subcortical, and cerebellar injections for the treatment of Niemann-Pick disease type A.
Bu J, Ashe KM, Bringas J, Marshall J, Dodge JC, Cabrera-Salazar MA, Forsayeth J, Schuchman EH, Bankiewicz KS, Cheng SH, Shihabuddin LS, Passini MA. Bu J, et al. Mol Ther. 2012 Oct;20(10):1893-901. doi: 10.1038/mt.2012.118. Epub 2012 Jul 24. Mol Ther. 2012. PMID: 22828503 Free PMC article. - Gene therapy for the neurological manifestations in lysosomal storage disorders.
Cheng SH. Cheng SH. J Lipid Res. 2014 Sep;55(9):1827-38. doi: 10.1194/jlr.R047175. Epub 2014 Mar 29. J Lipid Res. 2014. PMID: 24683200 Free PMC article. Review. - Acid Sphingomyelinase Deficiency: A Clinical and Immunological Perspective.
Pinto C, Sousa D, Ghilas V, Dardis A, Scarpa M, Macedo MF. Pinto C, et al. Int J Mol Sci. 2021 Nov 28;22(23):12870. doi: 10.3390/ijms222312870. Int J Mol Sci. 2021. PMID: 34884674 Free PMC article. Review.
Cited by
- Kinetics and MR-Based Monitoring of AAV9 Vector Delivery into Cerebrospinal Fluid of Nonhuman Primates.
Ohno K, Samaranch L, Hadaczek P, Bringas JR, Allen PC, Sudhakar V, Stockinger DE, Snieckus C, Campagna MV, San Sebastian W, Naidoo J, Chen H, Forsayeth J, Salegio EA, Hwa GGC, Bankiewicz KS. Ohno K, et al. Mol Ther Methods Clin Dev. 2018 Dec 8;13:47-54. doi: 10.1016/j.omtm.2018.12.001. eCollection 2019 Jun 14. Mol Ther Methods Clin Dev. 2018. PMID: 30666308 Free PMC article. - Lentiviral-mediated gene transfer to the sheep brain: implications for gene therapy in Batten disease.
Linterman KS, Palmer DN, Kay GW, Barry LA, Mitchell NL, McFarlane RG, Black MA, Sands MS, Hughes SM. Linterman KS, et al. Hum Gene Ther. 2011 Aug;22(8):1011-20. doi: 10.1089/hum.2011.026. Epub 2011 May 19. Hum Gene Ther. 2011. PMID: 21595499 Free PMC article. - Gene Therapy for the Treatment of Neurological Disorders: Metabolic Disorders.
Gessler DJ, Gao G. Gessler DJ, et al. Methods Mol Biol. 2016;1382:429-65. doi: 10.1007/978-1-4939-3271-9_30. Methods Mol Biol. 2016. PMID: 26611604 Free PMC article. Review. - Gene-based therapy of Parkinson's Disease: Translation from animal model to human clinical trial employing convection enhanced delivery.
Miranpuri GS, Kumbier L, Hinchman A, Schomberg D, Wang A, Marshall H, Kubota K, Ross C, Sillay K. Miranpuri GS, et al. Ann Neurosci. 2012 Jul;19(3):133-46. doi: 10.5214/ans.0972.7531.190310. Ann Neurosci. 2012. PMID: 25205986 Free PMC article. Review. - Rapid inverse planning for pressure-driven drug infusions in the brain.
Rosenbluth KH, Martin AJ, Mittermeyer S, Eschermann J, Dickinson PJ, Bankiewicz KS. Rosenbluth KH, et al. PLoS One. 2013;8(2):e56397. doi: 10.1371/journal.pone.0056397. Epub 2013 Feb 15. PLoS One. 2013. PMID: 23457563 Free PMC article.
References
- Bankiewicz K.S. Forsayeth J. Eberling J.L. Sanchez-Pernaute R. Pivirotto P. Bringas J. Herscovitch P. Carson R.E. Eckelman W. Reutter B. Cunningham J. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAVhAADC. Mol. Ther. 2006;14:564–570. - PubMed
- Barbon C.M. Ziegler R.J. Li C. Armentano D. Cherry M. Desnick R.J. Schuchman E.H. Cheng S.H. AAV8-mediated hepatic expression of acid sphingomyelinase corrects the metabolic defect in the visceral organs of a mouse model of Niemann-Pick disease. Mol. Ther. 2005;12:431–440. - PubMed
- Barranger J.M. Novelli E.A. Gene therapy for lysosomal storage disorders. Expert Opin. Biol. Ther. 2001;1:857–867. - PubMed
- Bartlett J.S. Samulski R.J. McCown T.J. Selective and rapid uptake of adeno-associated virus type 2 in brain. Hum. Gene Ther. 1998;9:1181–1186. - PubMed
- Barton N.W. Brady R.O. Dambrosia J.M. Di Bisceglie A.M. Doppelt S.H. Hill S.C. Mankin H.J. Murray G.J. Parker R.I. Argoff C.E. Grewal R.P. Yu K.-T. Replacement therapy for inherited enzyme deficiency: Macrophage-targeted glucocerebrosidase for Gaucher's disease. N. Engl. J. Med. 1991;324:1464–1470. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical