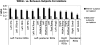New method for fMRI investigations of language: defining ROIs functionally in individual subjects - PubMed (original) (raw)
New method for fMRI investigations of language: defining ROIs functionally in individual subjects
Evelina Fedorenko et al. J Neurophysiol. 2010 Aug.
Abstract
Previous neuroimaging research has identified a number of brain regions sensitive to different aspects of linguistic processing, but precise functional characterization of these regions has proven challenging. We hypothesize that clearer functional specificity may emerge if candidate language-sensitive regions are identified functionally within each subject individually, a method that has revealed striking functional specificity in visual cortex but that has rarely been applied to neuroimaging studies of language. This method enables pooling of data from corresponding functional regions across subjects rather than from corresponding locations in stereotaxic space (which may differ functionally because of the anatomical variability across subjects). However, it is far from obvious a priori that this method will work as it requires that multiple stringent conditions be met. Specifically, candidate language-sensitive brain regions must be identifiable functionally within individual subjects in a short scan, must be replicable within subjects and have clear correspondence across subjects, and must manifest key signatures of language processing (e.g., a higher response to sentences than nonword strings, whether visual or auditory). We show here that this method does indeed work: we identify 13 candidate language-sensitive regions that meet these criteria, each present in >or=80% of subjects individually. The selectivity of these regions is stronger using our method than when standard group analyses are conducted on the same data, suggesting that the future application of this method may reveal clearer functional specificity than has been evident in prior neuroimaging research on language.
Figures
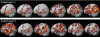
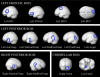
Fig. 1.
Sample activations in the left hemispheres of individual subjects for the sentences > nonwords contrast (top: sample subjects from experiment 1; bottom: sample subjects from experiment 2). Threshold: false discovery rate (FDR) 0.05.
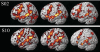
Fig. 2.
Activations in 2 sample subjects (S02, S10) for all of the runs (left: 8 runs in S02, 7 runs in S10), only the odd-numbered runs (middle), and only the even-numbered runs (right). [Four runs using the design of the experiments presented here take ∼30–40 min; however, this is because these runs include four experimental conditions. With just 2 conditions (sentences and nonwords), which is all that is necessary for functionally defining language-sensitive regions of interest (ROIs), only 2 runs are required, which take ∼15–20 min.]
Fig. 3.
Probabilistic overlap map for subjects in experiments 1 and 2 (n = 25). Colors indicate the number of subjects showing significant activation for sentences > nonwords in each voxel (the maximum possible value of a voxel equals the number of subjects included in the map, i.e., 25).
Fig. 4.
The relationship between the size of the group-level partitions (for all 180 partitions) and the number of subjects that have a nonzero intersection with the partition (i.e., ≥1 suprathreshold voxel within the borders of the partition). In dark gray are partitions that have a nonzero intersection with ≥80% of the subjects. In light gray are partitions that have a nonzero intersection with 60–79% of the subjects.
Fig. 5.
Two sample functional ROIs [fROIs, left inferior frontal gyrus (IFG) and left middle frontal gyrus (MFG)] in 7 sample subjects. The borders of the group-level partitions are shown in blue and the subject-specific activations are shown in red.
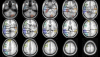
Fig. 6.
Group-level partitions corresponding to the 13 key fROIs shown on a slice mosaic.
Fig. 7.
Group-level partitions corresponding to the 13 key fROIs projected onto the brain surface.
Fig. 8.
Responses of the 13 fROIs to the sentences and nonwords conditions in an independent subset of the data in experiments 1 and 2 (1st 2 bars), in the 1st visual run in experiment 3 (2nd 2 bars), and in the auditory runs in experiment 3 (last 2 bars). Error bars represent SE.
Fig. 9.
Correlations across the voxels in each of the 13 group-level partitions comparing odd- vs. even-numbered runs within subjects (■) and comparing odd- vs. even-numbered runs between subjects ( ). Error bars represent standard errors of the mean.
). Error bars represent standard errors of the mean.
Fig. 10.
A comparison of the selectivity (the size of the sentences > nonwords effect) of group-constrained subject-specific (GcSS) fROIs vs. group-level fROIs (based on the random-effects group analysis of subjects in experiments 1 and 2, n = 25). Significance levels: left orbital IFG (IFGorb) < 0.05; left cerebellum < 0.01; left angular gyrus (AngG), left middle frontal (MFG), right cerebellum, left superior frontal gyrus (SFG) < 0.005; the rest of the regions <0.001. Error bars represent SE.
Similar articles
- Syntactic processing in the human brain: what we know, what we don't know, and a suggestion for how to proceed.
Fedorenko E, Nieto-Castañón A, Kanwisher N. Fedorenko E, et al. Brain Lang. 2012 Feb;120(2):187-207. doi: 10.1016/j.bandl.2011.01.001. Epub 2011 Feb 18. Brain Lang. 2012. PMID: 21334056 Free PMC article. Review. - The left occipitotemporal system in reading: disruption of focal fMRI connectivity to left inferior frontal and inferior parietal language areas in children with dyslexia.
van der Mark S, Klaver P, Bucher K, Maurer U, Schulz E, Brem S, Martin E, Brandeis D. van der Mark S, et al. Neuroimage. 2011 Feb 1;54(3):2426-36. doi: 10.1016/j.neuroimage.2010.10.002. Epub 2010 Oct 8. Neuroimage. 2011. PMID: 20934519 - The diminishing dominance of the dominant hemisphere: Language fMRI in focal epilepsy.
Tailby C, Abbott DF, Jackson GD. Tailby C, et al. Neuroimage Clin. 2017 Jan 16;14:141-150. doi: 10.1016/j.nicl.2017.01.011. eCollection 2017. Neuroimage Clin. 2017. PMID: 28180072 Free PMC article. - Human brain language processing areas identified by functional magnetic resonance imaging using a lexical decision task.
Calandra-Buonaura G, Basso G, Gorno-Tempini ML, Serafini M, Pagnoni G, Baraldi P, Porro CA, Nichelli P. Calandra-Buonaura G, et al. Funct Neurol. 2002 Oct-Dec;17(4):183-91. Funct Neurol. 2002. PMID: 12675261 - Dynamic changes in the mental rotation network revealed by pattern recognition analysis of fMRI data.
Mourao-Miranda J, Ecker C, Sato JR, Brammer M. Mourao-Miranda J, et al. J Cogn Neurosci. 2009 May;21(5):890-904. doi: 10.1162/jocn.2009.21078. J Cogn Neurosci. 2009. PMID: 18702583
Cited by
- A Bayesian optimization approach for rapidly mapping residual network function in stroke.
Lorenz R, Johal M, Dick F, Hampshire A, Leech R, Geranmayeh F. Lorenz R, et al. Brain. 2021 Aug 17;144(7):2120-2134. doi: 10.1093/brain/awab109. Brain. 2021. PMID: 33725125 Free PMC article. - Inter-individual, hemispheric and sex variability of brain activations during numerosity processing.
Zang Z, Chi X, Luan M, Hu S, Zhou K, Liu J. Zang Z, et al. Brain Struct Funct. 2024 Mar;229(2):459-475. doi: 10.1007/s00429-023-02747-3. Epub 2024 Jan 10. Brain Struct Funct. 2024. PMID: 38197958 Free PMC article. - Reading Reshapes Stimulus Selectivity in the Visual Word Form Area.
Chauhan VS, McCook KC, White AL. Chauhan VS, et al. eNeuro. 2024 Jul 26;11(7):ENEURO.0228-24.2024. doi: 10.1523/ENEURO.0228-24.2024. Print 2024 Jul. eNeuro. 2024. PMID: 38997142 Free PMC article. - Functional Network Dynamics of the Language System.
Chai LR, Mattar MG, Blank IA, Fedorenko E, Bassett DS. Chai LR, et al. Cereb Cortex. 2016 Oct 17;26(11):4148-4159. doi: 10.1093/cercor/bhw238. Cereb Cortex. 2016. PMID: 27550868 Free PMC article. - Lack of selectivity for syntax relative to word meanings throughout the language network.
Fedorenko E, Blank IA, Siegelman M, Mineroff Z. Fedorenko E, et al. Cognition. 2020 Oct;203:104348. doi: 10.1016/j.cognition.2020.104348. Epub 2020 Jun 20. Cognition. 2020. PMID: 32569894 Free PMC article.
References
- Amunts K, Willmes K. From intersubject variability in clinical syndromes to anatomical variability. Brain Lang 96: 147; discussion 157–1701177–150, 2006 - PubMed
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HB, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol 412: 319–341, 1999 - PubMed
- Ben-Shachar M, Hendler T, Kahn I, Ben-Bashat D, Grodzinsky Y. The neural reality of syntactic transformations: evidence from functional magnetic resonance imaging. Psychol Sci 14: 433–440, 2003 - PubMed
- Ben-Shachar M, Palti D, Grodzinsky Y. Neural correlates of syntactic movement: converging evidence from two fMRI experiments. Neuroimage 21: 1320–1336, 2004 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous