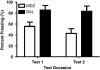Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory - PubMed (original) (raw)
Review
. 2010 Jul;35(8):1625-52.
doi: 10.1038/npp.2010.53. Epub 2010 Apr 21.
Affiliations
- PMID: 20410874
- PMCID: PMC3055480
- DOI: 10.1038/npp.2010.53
Review
Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory
Steve R Makkar et al. Neuropsychopharmacology. 2010 Jul.
Erratum in
- Neuropsychopharmacology. 2012 Jun;37(7):1793-4
Abstract
The current review systematically documents the role of gamma-amino-butyric acid (GABA) in different aspects of fear memory-acquisition and consolidation, reconsolidation, and extinction, and attempts to resolve apparent contradictions in the data in order to identify the function of GABA(A) receptors in fear memory. First, numerous studies have shown that pre- and post-training administration of drugs that facilitate GABAergic transmission disrupt the initial formation of fear memories, indicating a role for GABA(A) receptors, possibly within the amygdala and hippocampus, in the acquisition and consolidation of fear memories. Similarly, recent evidence indicates that these drugs are also detrimental to the restorage of fear memories after their reactivation. This suggests a role for GABA(A) receptors in the reconsolidation of fear memories, although the precise neural circuits are yet to be identified. Finally, research regarding the role of GABA in extinction has shown that GABAergic transmission is also disruptive to the formation of newly acquired extinction memories. We argue that contradictions to these patterns are the result of variations in (a) the location of drug infusion, (b) the dosage of the drug and/or (c) the time point of drug administration. The question of whether these GABA-induced memory deficits reflect deficits in retrieval is discussed. Overall, the evidence implies that the processes mediating memory stability consequent to initial fear learning, memory reactivation, and extinction training are dependent on a common mechanism of reduced GABAergic neurotransmission.
Figures
Figure 1
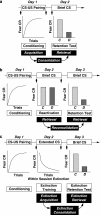
Schematics for the phenomena of acquisition (a), reconsolidation (b) and extinction (c). The first line in each schematic is procedural, with the arrows indicating usual points of drug administration in different published studies. The second line indicates what happens behaviorally, in particular the extent of fear responding by the control, C, and animals receiving the disruptive agent, D. In this case, the disruptive agent is the GABAA receptor agonist. The third line is the common paradigm nomenclature for that part of the procedure. The fourth line indicates the key assumed memory processes important to that particular phenomenon; preceding processes are assumed (eg, for reconsolidation and extinction, the initial acquisition process is assumed; for extinction, it may be that some reconsolidation occurs during the early phase of extinction training). It should be noted that the process of ‘retrieval' indicates access to the memory representation; additional processes that may then interfere with or facilitate CR expression are not explicitly indicated (nor are basic sensory processes that are assumed to be operative during acquisition). CR=conditioned response; CS=conditioned stimulus; US=unconditioned stimulus; brief CS=a limited number or duration of CS presentations; extended CS=many or long duration presentation of CS.
Figure 2
Hypothetical schematic model of the GABAA receptor channel, made of two _α_-, two _β_- and a single _γ_-subunit (ie, a 2 : 2 : 1 stoichiometry). Also displayed are the common GABAA receptor ligands described in this review, and their respective binding sites if known. Note that the GABA binding site is located at the junction between the alpha and beta subunits. Agonists such as muscimol, and antagonists such as bicuculline bind to this site. Neurosteroids may also bind to interfacial residues between the α- and _β_-subunits. Neurosteroids may additionally exert modulatory effects at _α_-subunit transmembrane domains. The benzodiazepine binding site is located at the interface of the _α_- and _γ_- subunits. Antagonists such as flumazenil and inverse agonists such as FG7142 also bind to this site. The precise binding site of barbiturates has not been identified. Non-competitive antagonists such as picrotoxin bind to distinct non-competitive sites located at the chloride ion channel.
Figure 3
Yee et al's (2004) schematic representation of the complex GABAergic circuits involved in the acquisition, consolidation, and expression of extinction, as well as the interaction between GABA and excitatory neurotransmitters, particularly glutamate binding to NMDA receptors, in the storage of extinction memories. The nodes illustrated in this diagram (circular nodes denoting glutamatergic units, and octagonal nodes denoting GABAergic units) represent assemblies or networks of neurons. The left hand side of the diagram demonstrates the formation of an association between a conditioned stimulus (CS) such as a tone, and an unconditioned stimulus (US) such as a shock. This excitatory learning is hypothesized to involve NMDA-mediated synaptic plasticity. GABAergic transmission may be involved in suppressing excitatory activity, thereby impairing the consolidation of fear memories. GABAergic antagonists and inverse agonists, which reduce GABAergic activity, would reduce such tonic inhibition, thus facilitating the acquisition and storage of the CS–US fear memory. The neural pathway of extinction is displayed on the right hand side. This involves the formation of links between nodes carrying information about the CS to GABAergic units, which leads to reduced expression of the conditioned response (CR) following CS presentation. In addition, representations related to detection of the absence of the US (as in extinction training) and sensory inputs carrying information about the extinction context are also hypothesized to connect to these GABAergic units which are involved in reducing CR output. These connections to the GABAergic units are strengthened by NMDA-dependent mechanisms during extinction learning, and these connections are themselves under the modulation of additional GABAergic interneurons. Therefore, the presence of GABAergic drugs during acquisition or consolidation phases of extinction would inhibit the excitatory neural processes (involving glutamate and NMDA receptors), which are critically involved in storing the extinction memory. In addition, it is evident that after extinction training, the expression of extinguished responding at test requires the activation of GABAergic units to suppress conditioned fear responding (bottom, right). The administration of GABAergic drugs during this phase of extinction would enhance inhibitory transmission, facilitating the expression of conditioned responding (eg, low levels of freezing, startle, heart rate). Copyright (2010) Wiley. Figure used with permission from Yee et al (2004), GABAA receptors containing the _α_5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear, Eur J Neurosci, John Wiley and Sons.
Figure 4
The effect of MDZ on the reconsolidation of auditory fear memory. Mean (±SEM) percentage freezing during tests 1 and 2 for rats administered MDZ (_n_=8) or saline (_n_=7). Test 1 took place 24 h after reactivation and test 2 took place 10 days after test 1. MDZ-treated rats display a deficit in freezing that is evident at both test sessions. MDZ=midazolam, SAL=saline (after reactivation).
Figure 5
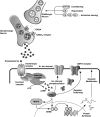
Hypothesized GABAergic molecular pathway of acquisition/consolidation, reconsolidation and extinction consolidation. After each critical procedural event (conditioning, reactivation, and extinction training, respectively; see Figure 1), there is a downregulation of GABAergic molecules within synapses, possibly in different areas of the amygdala, hippocampus and PFC, depending on the phenomenon. This leads to disinhibition of noradrenergic neurons, leading to elevated release of norepinephrine (NE). NE then binds to and activates _β_-adrenergic receptors. This triggers the intracellular activation of the enzyme adenylyl cyclase (by G-proteins), which initiates the formation of cyclic AMP (cAMP). cAMP then triggers upregulation of Protein Kinase A (PKA). PKA enhances cell excitability by the phosphorylating of receptors and ion channels (such as K+ channels), causing them to close. PKA also facilitates AMPA receptor trafficking. In addition, PKA together with MAPK stimulates the phosphorylation of various transcription factors such as CREB, which are involved in gene transcription and the activation of immediate early genes (IEGs). The products of gene transcription are used for protein synthesis. The proteins are then used for the synaptic modifications necessary for stabilization of the new memory, reactivated memory or the extinction trace. Abbreviations: AMPA=_α_-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate; ATP=adenosine triphosphate; cAMP=cyclic AMP; CREB=cAMP response element-binding protein; CS=conditioned stimulus; GABA=_γ_-amino butyric acid; MAPK=mitogen-activated protein kinase; PKA=Protein kinase A; US=unconditioned stimulus.
Similar articles
- [GABA-Receptors in Modulation of Fear Memory Extinction].
Dubrovina NI. Dubrovina NI. Zh Vyssh Nerv Deiat Im I P Pavlova. 2016 Mar-Apr;66(2):131-47. Zh Vyssh Nerv Deiat Im I P Pavlova. 2016. PMID: 27538279 Review. Russian. - Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala.
Berlau DJ, McGaugh JL. Berlau DJ, et al. Neurobiol Learn Mem. 2006 Sep;86(2):123-32. doi: 10.1016/j.nlm.2005.12.008. Epub 2006 Feb 3. Neurobiol Learn Mem. 2006. PMID: 16458544 - Memory consolidation and reconsolidation in an invertebrate model: the role of the GABAergic system.
Carbó Tano M, Molina VA, Maldonado H, Pedreira ME. Carbó Tano M, et al. Neuroscience. 2009 Jan 23;158(2):387-401. doi: 10.1016/j.neuroscience.2008.10.039. Epub 2008 Oct 30. Neuroscience. 2009. PMID: 19015009 - The endogenous cannabinoid system controls extinction of aversive memories.
Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B. Marsicano G, et al. Nature. 2002 Aug 1;418(6897):530-4. doi: 10.1038/nature00839. Nature. 2002. PMID: 12152079 - Erasing fear memories with extinction training.
Quirk GJ, Paré D, Richardson R, Herry C, Monfils MH, Schiller D, Vicentic A. Quirk GJ, et al. J Neurosci. 2010 Nov 10;30(45):14993-7. doi: 10.1523/JNEUROSCI.4268-10.2010. J Neurosci. 2010. PMID: 21068303 Free PMC article. Review.
Cited by
- Associative memory cells of encoding fear signals and anxiety are recruited by neuroligin-3-mediated synapse formation.
Chen B, Zhang Y, Xiao H, Wang L, Li J, Xu Y, Wang JH. Chen B, et al. Commun Biol. 2024 Nov 8;7(1):1464. doi: 10.1038/s42003-024-07170-w. Commun Biol. 2024. PMID: 39511365 Free PMC article. - MRS-assessed brain GABA modulation in response to task performance and learning.
Li H, Rodríguez-Nieto G, Chalavi S, Seer C, Mikkelsen M, Edden RAE, Swinnen SP. Li H, et al. Behav Brain Funct. 2024 Aug 31;20(1):22. doi: 10.1186/s12993-024-00248-9. Behav Brain Funct. 2024. PMID: 39217354 Free PMC article. Review. - Neurodevelopmental Disorders Associated with Gut Microbiome Dysbiosis in Children.
Borrego-Ruiz A, Borrego JJ. Borrego-Ruiz A, et al. Children (Basel). 2024 Jun 28;11(7):796. doi: 10.3390/children11070796. Children (Basel). 2024. PMID: 39062245 Free PMC article. Review. - Increased cortical inhibition following brief motor memory reactivation supports reconsolidation and overnight offline learning gains.
Eisenstein T, Furman-Haran E, Tal A. Eisenstein T, et al. Proc Natl Acad Sci U S A. 2023 Dec 26;120(52):e2303985120. doi: 10.1073/pnas.2303985120. Epub 2023 Dec 19. Proc Natl Acad Sci U S A. 2023. PMID: 38113264 Free PMC article. - Recent Progress in the Development of New Antiepileptic Drugs with Novel Targets.
Belete TM. Belete TM. Ann Neurosci. 2023 Oct;30(4):262-276. doi: 10.1177/09727531231185991. Epub 2023 Aug 17. Ann Neurosci. 2023. PMID: 38020406 Free PMC article. Review.
References
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11:180–187. - PubMed
- Akirav I. NMDA partial agonist reverses blocking of extinction of aversive memory by GABA (A) agonist in the amygdala. Neuropsychopharmacology. 2007;32:542–550. - PubMed
- Akirav I, Haizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABAA agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci. 2006;23:758–764. - PubMed
- Amaral OB, Luft T, Cammarota M, Izquierdo I, Roesler R. Temporary inactivation of the dorsal hippocampus induces a transient impairment in retrieval of aversive memory. Behavioral Brain Res. 2007;180:113–118. - PubMed
- Amin J, Weiss DS. GABAA receptor needs two homologous domains of the b subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical