Targeting the TGF-beta1 pathway to prevent normal tissue injury after cancer therapy - PubMed (original) (raw)
Review
Targeting the TGF-beta1 pathway to prevent normal tissue injury after cancer therapy
Mitchell S Anscher. Oncologist. 2010.
Abstract
With >10,000,000 cancer survivors in the U.S. alone, the late effects of cancer treatment are a significant public health issue. Over the past 15 years, much work has been done that has led to an improvement in our understanding of the molecular mechanisms underlying the development of normal tissue injury after cancer therapy. In many cases, these injuries are characterized at the histologic level by loss of parenchymal cells, excessive fibrosis, and tissue atrophy. Among the many cytokines involved in this process, transforming growth factor (TGF)-beta1 is thought to play a pivotal role. TGF-beta1 has a multitude of functions, including both promoting the formation and inhibiting the breakdown of connective tissue. It also inhibits epithelial cell proliferation. TGF-beta1 is overexpressed at sites of injury after radiation and chemotherapy. Thus, TGF-beta1 represents a logical target for molecular therapies designed to prevent or reduce normal tissue injury after cancer therapy. Herein, the evidence supporting the critical role of TGF-beta1 in the development of normal tissue injury after cancer therapy is reviewed and the results of recent research aimed at preventing normal tissue injury by targeting the TGF-beta1 pathway are presented.
Conflict of interest statement
Disclosures: Mitchell S. Anscher: Consultant/advisory role: Civa Tech.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers.
Figures
Figure 1.
TGF-β expression in radiation-induced lung injury. (A): Radiation (RT)-induced lung injury in a rat model. Fischer 344 rats were irradiated to 28 Gy in one fraction to the right hemithorax and sacrificed at 6 weeks or 6 months after RT. Slides were stained with either hematoxylin & eosin (top row) or Masson's trichrome (bottom row). A nonirradiated control lung (left column) demonstrates the normal honeycomb architecture of the lung. At 6 weeks after irradiation (middle column), one begins to see thickening of the alveolar walls resulting from edema, corresponding to the inflammatory phase, but with little fibrosis. At 6 months (right column), there is complete loss of normal alveolar architecture, with extensive fibrosis (green staining in bottom right panel). Reprinted from Stone HB, Coleman CN, Anscher MS et al. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol 2003;4:529–536, with permission from Elsevier. (B): Fischer 344 rats were irradiated to 28 Gy in one fraction to the right hemithorax, sacrificed 6 months after radiation, and stained for transforming growth factor (TGF)-β1. There is very little staining for TGF-β1 in the control lung (nonirradiated) in contrast to the irradiated lung, which demonstrates greater expression of TGF-β1 in regions of fibrosis.
Figure 2.
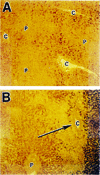
TGF-β expression in chemotherapy-induced liver injury. Section of normal liver (A) and liver from patient that died of hepatic veno-occlusive disease after high-dose chemotherapy (B). In ( B ), evidence of extensive fibrosis around the central veins (C) is noted (arrow) and there is greater staining for transforming growth factor β1 (reddish brown areas) immediately adjacent to regions of fibrosis than in the normal liver in ( A ). P, portal vein. Reprinted from Anscher MS, Kong FM, Jirtle RL. The relevance of transforming growth factor beta 1 in pulmonary injury after radiation therapy. Lung Cancer 1998;19:109–120, with permission from Elsevier.
Figure 3.
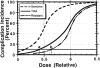
Altering dose on the basis of an individual patient's sensitivity to toxicity can affect the therapeutic ratio. Dosing of chemotherapy or radiation is based on sensitivity to toxicity that is based on population averages (solid line). In reality, any population also contains individuals that will be either more sensitive (dashed line) or more resistant (dot-dash line) to treatment toxicity than the average population. The sensitive patients, which probably comprise ≤5%–10% of the overall population [1, 2], nonetheless drive the dosing schemes. In this example, by holding the acceptable complication incidence at 10%, a resistant patient (b) could receive an approximately 5% greater dose than an average patient (a) and a 10% greater dose than a sensitive patient. Dose differences of this magnitude have been associated with differences in outcome.
Figure 4.
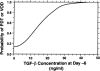
Correlation of plasma transforming growth factor (TGF)-β1 concentration and the risk for pulmonary drug toxicity (PDT) or hepatic veno-occlusive disease (VOD) after high-dose chemotherapy for advanced breast cancer. Blood samples were taken from patients after induction chemotherapy but prior to administration of high-dose chemotherapy (day −6) and bone marrow transplant. There is a strong correlation between the plasma TGF-β1 concentration at day −6 and the risk for either PDT or VOD developing after subsequent high-dose chemotherapy. Reprinted from Anscher MS, Kong FM, Jirtle RL. The relevance of transforming growth factor beta 1 in pulmonary injury after radiation therapy. Lung Cancer 1998;19:109–120, with permission from Elsevier.
Figure 5.
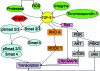
Potential targets for intervention in the TGF-β1 pathway (partial list) [17, 74, 114]. The inactive form of TGF-β1 can be activated through the action of several factors (green ovals), including ROS, proteases, integrins, and thrombospondin-1. The active form of TGF-β1 (yellow) can then signal through either Smad-dependent (light blue) or Smad-independent pathways (orange, pink, gray). Any point along these pathways might be targeted by potential inhibitors. Abbreviations: ERK, extracellular signal–related kinase; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3′ kinase; ROS, reactive oxygen species; TGF, transforming growth factor.
Similar articles
- CpG-oligodeoxynucleotides may be effective for preventing ionizing radiation induced pulmonary fibrosis.
Zhang C, Zhao H, Li BL, Fu-Gao, Liu H, Cai JM, Zheng M. Zhang C, et al. Toxicol Lett. 2018 Aug;292:181-189. doi: 10.1016/j.toxlet.2018.04.009. Epub 2018 Apr 19. Toxicol Lett. 2018. PMID: 29679710 - Role of phosphatidylinositol 3-kinase signaling pathway in radiation-induced liver injury.
Xiao L, Zhang H, Yang X, Mahati S, Wu G, Xiaheding Y, Bao YX, Xiao H. Xiao L, et al. Kaohsiung J Med Sci. 2020 Dec;36(12):990-997. doi: 10.1002/kjm2.12279. Epub 2020 Jul 30. Kaohsiung J Med Sci. 2020. PMID: 32729224 - Specific signals involved in the long-term maintenance of radiation-induced fibrogenic differentiation: a role for CCN2 and low concentration of TGF-beta1.
Haydont V, Riser BL, Aigueperse J, Vozenin-Brotons MC. Haydont V, et al. Am J Physiol Cell Physiol. 2008 Jun;294(6):C1332-41. doi: 10.1152/ajpcell.90626.2007. Epub 2008 Apr 9. Am J Physiol Cell Physiol. 2008. PMID: 18400984 - Role of Platelet-Derived Transforming Growth Factor-β1 and Reactive Oxygen Species in Radiation-Induced Organ Fibrosis.
Ahamed J, Laurence J. Ahamed J, et al. Antioxid Redox Signal. 2017 Nov 1;27(13):977-988. doi: 10.1089/ars.2017.7064. Epub 2017 Jul 5. Antioxid Redox Signal. 2017. PMID: 28562065 Free PMC article. Review. - Negative regulators of TGF-β1 signaling in renal fibrosis; pathological mechanisms and novel therapeutic opportunities.
Gifford CC, Tang J, Costello A, Khakoo NS, Nguyen TQ, Goldschmeding R, Higgins PJ, Samarakoon R. Gifford CC, et al. Clin Sci (Lond). 2021 Jan 29;135(2):275-303. doi: 10.1042/CS20201213. Clin Sci (Lond). 2021. PMID: 33480423 Review.
Cited by
- Nicotinamide Mononucleotide Supplementation Alleviates Doxorubicin-Induced Multi-Organ Fibrosis.
Wen F, Xu A, Wei W, Yang S, Xi Z, Ge Y, Wu S, Ju Z. Wen F, et al. Int J Mol Sci. 2024 May 13;25(10):5303. doi: 10.3390/ijms25105303. Int J Mol Sci. 2024. PMID: 38791345 Free PMC article. - Radiation Therapy and Myeloid-Derived Suppressor Cells: Breaking Down Their Cancerous Partnership.
Bergerud KMB, Berkseth M, Pardoll DM, Ganguly S, Kleinberg LR, Lawrence J, Odde DJ, Largaespada DA, Terezakis SA, Sloan L. Bergerud KMB, et al. Int J Radiat Oncol Biol Phys. 2024 May 1;119(1):42-55. doi: 10.1016/j.ijrobp.2023.11.050. Epub 2023 Nov 30. Int J Radiat Oncol Biol Phys. 2024. PMID: 38042450 Free PMC article. Review. - Smad3 promotes adverse cardiovascular remodeling and dysfunction in doxorubicin-treated hearts.
Cobb MS, Tao S, Shortt K, Girgis M, Hauptman J, Schriewer J, Chin Z, Dorfman E, Campbell K, Heruth DP, Shohet RV, Dawn B, Konorev EA. Cobb MS, et al. Am J Physiol Heart Circ Physiol. 2022 Dec 1;323(6):H1091-H1107. doi: 10.1152/ajpheart.00312.2022. Epub 2022 Oct 21. Am J Physiol Heart Circ Physiol. 2022. PMID: 36269647 Free PMC article. - Liquid biopsy in NSCLC: a new challenge in radiation therapy.
Perillo A, Olufemi MVA, De Robbio J, Mancuso RM, Roscigno A, Tirozzi M, Scognamiglio IR. Perillo A, et al. Explor Target Antitumor Ther. 2021;2(2):156-173. doi: 10.37349/etat.2021.00038. Epub 2021 Apr 30. Explor Target Antitumor Ther. 2021. PMID: 36046142 Free PMC article. Review. - The effects of apigenin administration on the inhibition of inflammatory responses and oxidative stress in the lung injury models: a systematic review and meta-analysis of preclinical evidence.
Rahimi A, Alimohammadi M, Faramarzi F, Alizadeh-Navaei R, Rafiei A. Rahimi A, et al. Inflammopharmacology. 2022 Aug;30(4):1259-1276. doi: 10.1007/s10787-022-00994-0. Epub 2022 Jun 4. Inflammopharmacology. 2022. PMID: 35661071 Review.
References
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. - PubMed
- Milano MT, Constine LS, Okunieff P. Normal tissue tolerance dose metrics for radiation therapy of major organs. Semin Radiat Oncol. 2007;17:131–140. - PubMed
- Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: Pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys. 2005;63:5–24. - PubMed
- Kong FM, Pan C, Eisbruch A, et al. Physical models and simpler dosimetric descriptors of radiation late toxicity. Semin Radiat Oncol. 2007;17:108–120. - PubMed
- Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: Mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys. 2006;66:1281–1293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous