Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity - PubMed (original) (raw)
Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity
Frank Weinberg et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Otto Warburg's theory on the origins of cancer postulates that tumor cells have defects in mitochondrial oxidative phosphorylation and therefore rely on high levels of aerobic glycolysis as the major source of ATP to fuel cellular proliferation (the Warburg effect). This is in contrast to normal cells, which primarily utilize oxidative phosphorylation for growth and survival. Here we report that the major function of glucose metabolism for Kras-induced anchorage-independent growth, a hallmark of transformed cells, is to support the pentose phosphate pathway. The major function of glycolytic ATP is to support growth under hypoxic conditions. Glutamine conversion into the tricarboxylic acid cycle intermediate alpha-ketoglutarate through glutaminase and alanine aminotransferase is essential for Kras-induced anchorage-independent growth. Mitochondrial metabolism allows for the generation of reactive oxygen species (ROS) which are required for Kras-induced anchorage-independent growth through regulation of the ERK MAPK signaling pathway. We show that the major source of ROS generation required for anchorage-independent growth is the Q(o) site of mitochondrial complex III. Furthermore, disruption of mitochondrial function by loss of the mitochondrial transcription factor A (TFAM) gene reduced tumorigenesis in an oncogenic Kras-driven mouse model of lung cancer. These results demonstrate that mitochondrial metabolism and mitochondrial ROS generation are essential for Kras-induced cell proliferation and tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
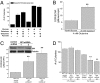
Fig. 1.
The pentose phosphate pathway, not glycolysis, is essential for Kras-induced growth under aerobic conditions. (A) Analysis of soft agar colonies of HCT116 cells, in media containing combinations of 20 mM glucose, 20 mM galactose, 4 mM glutamine, and 1 mM sodium pyruvate. (B) Ratio between oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) of HCT116 cells incubated with 4 mM glutamine plus 20 mM glucose or galactose. Mean ± SE (n = 20). **P < 0.01 (C) Ratio between OCR and ECAR of control and GPI shRNA #1 and #2 in HCT116 cells. Mean ± SE (n = 20). **P < 0.01 (D) Analysis of soft agar colonies of HCT116 expressing control or GPI shRNAs grown under normoxia (21% O2) or hypoxia (3% O2). Bars represent the mean ± SE (n = 16). **P < 0.01.
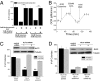
Fig. 2.
Glutamine catabolism by mitochondria is required for oncogenic Kras-induced anchorage-independent growth. (A) Analysis of soft agar colonies of HCT116 cells in media containing 4 mM glutamine with 20 mM glucose or 20 mM galactose in the presence of 2 mM aminooxyacetic acid (AOA) and 7 mM dimethyl α-ketoglutarate (DMK). Mean ± SE (n = 9). **P < 0.01. (B) Oxygen consumption rate (OCR) was measured in HCT116 cells incubated in basal media and subsequently exposed to glutamine, AOA, and DMK. (C) Analysis of soft agar colonies of HCT116 cells expressing control and alanine amiotransferase 2 (ALT2) shRNA #1 and #2 ± DMK or (D) control and glutaminase 1 (GLS1) shRNA #1 and #2 ± DMK. Bars represent the mean ± SE (n = 16 in C and n = 8 in D). **P < 0.01.
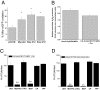
Fig. 3.
Mitochondrial ROS are required for oncogene-induced anchorage-independent growth. (A) Levels of oxidized mito-roGFP in p53DN, Myr-Akt-p53DN, HrasV12-p53DN, and KrasV12-p53DN cells. Mean ± SE (n = 4). *P < 0.05; Statistical comparisons were made between p53DN cells and Myr-Akt, HrasV12, or KrasV12 cells. (B) Intracellular H2O2 levels were assessed by Amplex Red in cell lysates of p53DN, KrasV12-p53DN cells, LSL KrasG12D 3T3 ± Cre. Mean ± SE (n = 4). Data represented is KrasV12-p53DN cells/immortalized p53DN and LSLKrasG12D3T3 +Cre/immortalized LSLKrasG12D. Analysis of soft agar colonies of (C) LSL-Kras G12D, and (D) HCT116 cell lines treated with mitochondrial targeted antioxidants 1 μM MCTPO, 1 μM MCP, or the control compounds 1 μM CTPO, 1 μM CP, and 1 μM TPP. Mean ± SE (n = 9). **P < 0.01; Statistical comparisons are between MCTPO or MCP and TPP.
Fig. 4.
Mitochondrial ROS regulate anchorage-independent growth through the MAPK/ERK1/2 pathway. (A) Effects of mitochondrial targeted nitroxides and control compounds on LSL-Kras G12D 3T3 MEFs + Cre cellular proliferation at 24, 48, or 72 hours after treatment. *P < 0.05; **P < 0.01. Statistical comparisons are between MCTPO or MCP and TPP. (B) Western blot analysis of phosphorylated ERK1/2 and Total ERK in LSL-KrasG12D 3T3 MEFs cell lysates serum starved for 18 hours (0 time point) or after 15 min serum stimulation post-48-hours treatment with 1 μM MCTPO, 1 μM CTPO, 1 μM MCP, 1 μM CP, and 1 μM TPP. (C) Western blot analysis of phosphorylated ERK1/2 and Total ERK 48 hours after treatment with no drug or 1 μM MCP in the presence of either 0 nM, 100 nM, or 500 nM U0126. (D) Analysis of soft agar colonies of LSL-KrasG12D 3T3 MEFs treated with either 0 or 1 μM MCP in the presence of 0 nM, 100 nM, or 500 nM UO216. Mean ± SE (n = 9). **P < 0.01. Statistical comparison was made between cells treated with Mito CP and cells not treated with Mito CP.
Fig. 5.
Mitochondrial complex III generated ROS are required for anchorage-independent growth. (A) Levels of oxidized mito-roGFP in 143B cells, ρ°143B cells, wild-type 143B cybrids, and Δcytochrome b 143B cybrids. (B) Analysis of soft agar colonies of 143B, ρ0143B cells, wild-type cybrids and Δcytochrome b cybrids. Mean ± SE (n = 9). *P < 0.05; **P < 0.01. (C) Analysis of soft agar colonies of wild-type cybrids and Δcytochrome b cybrids treated with 2 mM aminooxyacetic acid (AOA) ± 7 mM dimethyl α-ketoglutarate (DMK). (D) Western blot analysis of RISP protein and (E) analysis of soft agar colonies of wild-type 143B cybrids and Δcytochrome b 143B cybrids stably infected with negative control shRNA or RISP shRNA. **P < 0.01.
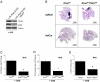
Fig. 6.
Mitochondrial metabolism is required for oncogenic Kras-driven mouse lung adenocarcinoma. (A) Western blot analysis of TFAM, cytochrome c oxidase subunit I protein (COXI), and α-TUBULIN in lung lysates and (B) histology of LSL-Kras G12Dfl/+ and LSL-Kras G12Dfl/+ Tfamfl/fl lungs 12-weeks post treatment with intratracheal instillation of Cre-recombinase. (Scale bars, 1 cm.) (C and D) Tumor loads and number of lesions per area of lung tissue in LSL-Kras G12Dfl/+ and LSL-Kras G12Dfl/+ Tfamfl/fl mice. Bars represent the mean ± SE (n = 6 animals). *P < 0.05. (E) Ki67 staining (proliferative index) of the LSL-Kras G12Dfl/+ and LSL-Kras G12Dfl/+ Tfamfl/fl mice lungs. Bars represent the mean ± SE (n = 6 animals). *P < 0.05.
Similar articles
- The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism.
Lu J, Tan M, Cai Q. Lu J, et al. Cancer Lett. 2015 Jan 28;356(2 Pt A):156-64. doi: 10.1016/j.canlet.2014.04.001. Epub 2014 Apr 13. Cancer Lett. 2015. PMID: 24732809 Free PMC article. Review. - Deletion of Pim kinases elevates the cellular levels of reactive oxygen species and sensitizes to K-Ras-induced cell killing.
Song JH, An N, Chatterjee S, Kistner-Griffin E, Mahajan S, Mehrotra S, Kraft AS. Song JH, et al. Oncogene. 2015 Jul;34(28):3728-36. doi: 10.1038/onc.2014.306. Epub 2014 Sep 22. Oncogene. 2015. PMID: 25241892 Free PMC article. - Drp1 Promotes KRas-Driven Metabolic Changes to Drive Pancreatic Tumor Growth.
Nagdas S, Kashatus JA, Nascimento A, Hussain SS, Trainor RE, Pollock SR, Adair SJ, Michaels AD, Sesaki H, Stelow EB, Bauer TW, Kashatus DF. Nagdas S, et al. Cell Rep. 2019 Aug 13;28(7):1845-1859.e5. doi: 10.1016/j.celrep.2019.07.031. Cell Rep. 2019. PMID: 31412251 Free PMC article. - KRAS-regulated glutamine metabolism requires UCP2-mediated aspartate transport to support pancreatic cancer growth.
Raho S, Capobianco L, Malivindi R, Vozza A, Piazzolla C, De Leonardis F, Gorgoglione R, Scarcia P, Pezzuto F, Agrimi G, Barile SN, Pisano I, Reshkin SJ, Greco MR, Cardone RA, Rago V, Li Y, Marobbio CMT, Sommergruber W, Riley CL, Lasorsa FM, Mills E, Vegliante MC, De Benedetto GE, Fratantonio D, Palmieri L, Dolce V, Fiermonte G. Raho S, et al. Nat Metab. 2020 Dec;2(12):1373-1381. doi: 10.1038/s42255-020-00315-1. Epub 2020 Nov 23. Nat Metab. 2020. PMID: 33230296 - Targeting metabolic reprogramming in KRAS-driven cancers.
Kawada K, Toda K, Sakai Y. Kawada K, et al. Int J Clin Oncol. 2017 Aug;22(4):651-659. doi: 10.1007/s10147-017-1156-4. Epub 2017 Jun 24. Int J Clin Oncol. 2017. PMID: 28647837 Review.
Cited by
- Combination of miR‑143 and miR‑506 reduces lung and pancreatic cancer cell growth through the downregulation of cyclin‑dependent kinases.
Hossian AKMN, Mackenzie GG, Mattheolabakis G. Hossian AKMN, et al. Oncol Rep. 2021 Apr;45(4):2. doi: 10.3892/or.2021.7953. Epub 2021 Mar 2. Oncol Rep. 2021. PMID: 33649787 Free PMC article. - Natural Agents Targeting Mitochondria in Cancer.
Mani S, Swargiary G, Singh KK. Mani S, et al. Int J Mol Sci. 2020 Sep 23;21(19):6992. doi: 10.3390/ijms21196992. Int J Mol Sci. 2020. PMID: 32977472 Free PMC article. Review. - MYC inhibition induces metabolic changes leading to accumulation of lipid droplets in tumor cells.
Zirath H, Frenzel A, Oliynyk G, Segerström L, Westermark UK, Larsson K, Munksgaard Persson M, Hultenby K, Lehtiö J, Einvik C, Påhlman S, Kogner P, Jakobsson PJ, Henriksson MA. Zirath H, et al. Proc Natl Acad Sci U S A. 2013 Jun 18;110(25):10258-63. doi: 10.1073/pnas.1222404110. Epub 2013 Jun 3. Proc Natl Acad Sci U S A. 2013. PMID: 23733953 Free PMC article. - Mechanisms of superoxide signaling in epigenetic processes: relation to aging and cancer.
Afanas'ev I. Afanas'ev I. Aging Dis. 2015 Jun 1;6(3):216-27. doi: 10.14336/AD.2014.0924. eCollection 2015 Jun. Aging Dis. 2015. PMID: 26029480 Free PMC article. Review. - Metabolism of immune cells in cancer.
Leone RD, Powell JD. Leone RD, et al. Nat Rev Cancer. 2020 Sep;20(9):516-531. doi: 10.1038/s41568-020-0273-y. Epub 2020 Jul 6. Nat Rev Cancer. 2020. PMID: 32632251 Free PMC article. Review.
References
- Warburg O, Posener K, Negelein E. Uber den stoffwechsel der carcinomzelle. Biochem Z. 1924;152:309.
- Warburg O. On the origin of cancer cells. Science (New York) 1956;123:309–314. - PubMed
- Weinhouse S. On respiratory impairment in cancer cells. (Translated from eng) Science (New York) 1956;124:267–269. - PubMed
- Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Natl Rev. 2008;8:705–713. - PubMed
- Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: A genetic and biochemical update. Natl Rev. 2005;5:857–866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ES013995/ES/NIEHS NIH HHS/United States
- R01 ES013995/ES/NIEHS NIH HHS/United States
- CA125112/CA/NCI NIH HHS/United States
- R01CA123067-04/CA/NCI NIH HHS/United States
- T32 CA009560/CA/NCI NIH HHS/United States
- I01 BX000201/BX/BLRD VA/United States
- P01 HL071643/HL/NHLBI NIH HHS/United States
- R01 CA125112/CA/NCI NIH HHS/United States
- P01HL071643/HL/NHLBI NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- R01 ES015024/ES/NIEHS NIH HHS/United States
- R01 CA123067/CA/NCI NIH HHS/United States
- T32CA009560-22/CA/NCI NIH HHS/United States
- R01 CA152810/CA/NCI NIH HHS/United States
- T32 CA070085/CA/NCI NIH HHS/United States
- ES015024/ES/NIEHS NIH HHS/United States
- T32CA070085-13/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous