Charge as a selection criterion for translocation through the nuclear pore complex - PubMed (original) (raw)
Charge as a selection criterion for translocation through the nuclear pore complex
Lucy J Colwell et al. PLoS Comput Biol. 2010.
Abstract
Nuclear pore complexes (NPCs) are highly selective filters that control the exchange of material between nucleus and cytoplasm. The principles that govern selective filtering by NPCs are not fully understood. Previous studies find that cellular proteins capable of fast translocation through NPCs (transport receptors) are characterized by a high proportion of hydrophobic surface regions. Our analysis finds that transport receptors and their complexes are also highly negatively charged. Moreover, NPC components that constitute the permeability barrier are positively charged. We estimate that electrostatic interactions between a transport receptor and the NPC result in an energy gain of several k(B)T, which would enable significantly increased translocation rates of transport receptors relative to other cellular proteins. We suggest that negative charge is an essential criterion for selective passage through the NPC.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
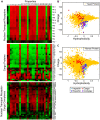
Figure 1. Nuclear transport receptors are more negatively charged than the majority of cellular proteins.
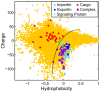
A. Heat map of the physical properties of transport receptors, cargo proteins, and transport receptor-cargo complexes from S.c. Each row corresponds to a different protein or complex (Table S1), and each column to a different property (Table S2). The value of each property was obtained as described in the main text. Similar proteins are clustered using a Euclidean distance metric. The profiles of transport receptors resemble each other, but differ from those of cargo proteins. B. Reduced representation using the first principal component of the 27 hydrophobicity scales, and net charge at intracellular pH. We compare importins (light blue squares), exportins (dark blue squares), cargo proteins (red circles), and transport receptor-cargo complexes (purple triangles) from S.c. with the entire S.c. proteome (yellow circles). Transport receptors are characterized by high hydrophobicity and net negative charge, and reside at the edge of the S.c. proteome. In contrast, most cargos have comparably low hydrophobicity and net positive charge. Note that the light blue square corresponding to importinα (marked by an asterisk) falls in the region occupied by transport receptor-cargo complexes: indeed, efficient translocation of importinα requires binding to the transport receptors importinβ or CAS, respectively C. Reduced representation of proteins from H.s. We compare importins (light blue squares), exportins (dark blue squares), cargo proteins (red circles), and transport receptor-cargo complexes (purple triangles) with the entire H.s. proteome (yellow circles).
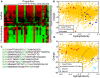
Figure 2. Most components of the NPC selectivity barrier are characterized by net positive charge.
A. The physical properties of S.c. nucleoporins displayed as a heat map. Each column represents a different property (Table S2), and each row a nucleoporin (Table S3). The value of each property was obtained as described in the main text. Clustering the proteins using a Euclidean metric separates them into two groups, corresponding to scaffold and FG-nucleoporins , . B. 2D property space representation of S.c. nucleoporins. The hydrophobicity index is plotted against net charge at pH 7.2. The scaffold nucleoporins (blue circles) are relatively hydrophobic, while the FG-nucleoporins (red circles) are characterized by low hydrophobicity and net positive charge. Yellow circles represent the S.c. proteome. C. An FG-rich domain from the nucleoporin Nup100. Like other FG-nucleoporins, Nup100 contains largely unstructured FG-domains that contain numerous short hydrophobic FG-repeats separated by hydrophilic, positively-charged linkers. D. Comparison of the unstructured FG-domains (red circles) with transport receptors (light and dark blue squares), and the S.c. proteome (yellow circles). The unstructured FG-domains and the transport receptors have complementary properties; the former have low hydrophobicity and mostly net positive charge, the latter have high hydrophobicity and net negative charge at pH 7.2. This suggests a role for electrostatic interactions between transport receptors and the unstructured FG-domains in the translocation reaction.
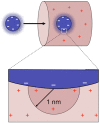
Figure 3. Strategy to estimate the electrostatic interactions between transport receptors and selective barrier components.
The interaction between one charge on the surface of the receptor with those nucleoporin charges within a screening length of 1nm (QNPC) is determined. This interaction is then scaled up to include the transport receptor's total charge (QNTR), given by the sum of its charged residues. See main text for details.
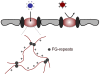
Figure 4. Charge as a selection criterion for nuclear transport.
The unfolded domains that constitute the permeability barrier are positively charged (red lines) and generate a positive charge density within the barrier. Particles with negative surface charge (e.g. transport receptors) adsorb to the positively charged nucleoporin domains and thereby selectively partition into the permeability barrier. In contrast, most soluble cellular proteins lack net negative charge; their entry into the NPC is energetically highly unfavorable.
Figure 5. Charge and hydrophobicity of a selection of signaling proteins from H.s. (listed in Table S4) (green diamonds) in comparison with importins (light blue squares), exportins (dark blue squares), cargo proteins (red circles), transport receptor-cargo complexes (purple triangles), and the H.s. proteome (yellow circles).
Signaling proteins that fall into the transport receptor-cargo complex regime (bottom right quadrant) are predicted by this analysis to efficiently self-translocate through the NPC without specific association to transport receptors. Note that beta-catenin (marked with asterisk), one example of a large protein that translocates without binding to a transport receptor, falls in this regime.
Similar articles
- Effect of charge, hydrophobicity, and sequence of nucleoporins on the translocation of model particles through the nuclear pore complex.
Tagliazucchi M, Peleg O, Kröger M, Rabin Y, Szleifer I. Tagliazucchi M, et al. Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):3363-8. doi: 10.1073/pnas.1212909110. Epub 2013 Feb 12. Proc Natl Acad Sci U S A. 2013. PMID: 23404701 Free PMC article. - Nucleoporins' exclusive amino acid sequence features regulate their transient interaction with and selectivity of cargo complexes in the nuclear pore.
Peyro M, Dickson AM, Mofrad MRK. Peyro M, et al. Mol Biol Cell. 2021 Nov 1;32(21):ar31. doi: 10.1091/mbc.E21-04-0161. Epub 2021 Sep 2. Mol Biol Cell. 2021. PMID: 34473567 Free PMC article. - Systematic analysis of barrier-forming FG hydrogels from Xenopus nuclear pore complexes.
Labokha AA, Gradmann S, Frey S, Hülsmann BB, Urlaub H, Baldus M, Görlich D. Labokha AA, et al. EMBO J. 2013 Jan 23;32(2):204-18. doi: 10.1038/emboj.2012.302. Epub 2012 Nov 30. EMBO J. 2013. PMID: 23202855 Free PMC article. - Nuclear pore complex biogenesis.
Fernandez-Martinez J, Rout MP. Fernandez-Martinez J, et al. Curr Opin Cell Biol. 2009 Aug;21(4):603-12. doi: 10.1016/j.ceb.2009.05.001. Epub 2009 Jun 11. Curr Opin Cell Biol. 2009. PMID: 19524430 Free PMC article. Review. - Functional architecture of the nuclear pore complex.
Grossman E, Medalia O, Zwerger M. Grossman E, et al. Annu Rev Biophys. 2012;41:557-84. doi: 10.1146/annurev-biophys-050511-102328. Annu Rev Biophys. 2012. PMID: 22577827 Review.
Cited by
- Brownian dynamics simulation of nucleocytoplasmic transport: a coarse-grained model for the functional state of the nuclear pore complex.
Moussavi-Baygi R, Jamali Y, Karimi R, Mofrad MR. Moussavi-Baygi R, et al. PLoS Comput Biol. 2011 Jun;7(6):e1002049. doi: 10.1371/journal.pcbi.1002049. Epub 2011 Jun 2. PLoS Comput Biol. 2011. PMID: 21673865 Free PMC article. - Probing High Permeability of Nuclear Pore Complexes by Scanning Electrochemical Microscopy: Ca2+ Effects on Transport Barriers.
Pathirathna P, Balla RJ, Jantz DT, Kurapati N, Gramm ER, Leonard KC, Amemiya S. Pathirathna P, et al. Anal Chem. 2019 Apr 16;91(8):5446-5454. doi: 10.1021/acs.analchem.9b00796. Epub 2019 Apr 3. Anal Chem. 2019. PMID: 30907572 Free PMC article. - Exploring the mechanism of plasmid DNA nuclear internalization with polymer-based vehicles.
Grandinetti G, Reineke TM. Grandinetti G, et al. Mol Pharm. 2012 Aug 6;9(8):2256-67. doi: 10.1021/mp300142d. Epub 2012 Jul 9. Mol Pharm. 2012. PMID: 22715912 Free PMC article. - Physical motif clustering within intrinsically disordered nucleoporin sequences reveals universal functional features.
Ando D, Colvin M, Rexach M, Gopinathan A. Ando D, et al. PLoS One. 2013 Sep 16;8(9):e73831. doi: 10.1371/journal.pone.0073831. eCollection 2013. PLoS One. 2013. PMID: 24066078 Free PMC article. - The particle in the spider's web: transport through biological hydrogels.
Witten J, Ribbeck K. Witten J, et al. Nanoscale. 2017 Jun 22;9(24):8080-8095. doi: 10.1039/c6nr09736g. Nanoscale. 2017. PMID: 28580973 Free PMC article. Review.
References
- Schwoebel ED, Talcott B, Cushman I, Moore MS. Ran-dependent signal-mediated nuclear import does not require GTP hydrolysis by Ran. J Biol Chem. 1998;273:35170–35175. - PubMed
- Englmeier L, Olivo JC, Mattaj IW. Receptor-mediated substrate translocation through the nuclear pore complex without nucleotide triphosphate hydrolysis. Curr Biol. 1999;9:30–41. - PubMed
- Ribbeck K, Kutay U, Paraskeva E, Görlich D. The translocation of transportin-cargo complexes through nuclear pores is independent of both Ran and energy. Curr Biol. 1999;9:47–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases