Internal representation of hierarchical sequences involves the default network - PubMed (original) (raw)
Internal representation of hierarchical sequences involves the default network
Baxter P Rogers et al. BMC Neurosci. 2010.
Abstract
Background: The default network is a set of brain regions that exhibit a reduction in BOLD response during attention-demanding cognitive tasks, and distinctive patterns of functional connectivity that typically include anti-correlations with a fronto-parietal network involved in attention, working memory, and executive control. The function of the default network regions has been attributed to introspection, self-awareness, and theory of mind judgments, and some of its regions are involved in episodic memory processes.
Results: Using the method of psycho-physiological interactions, we studied the functional connectivity of several regions in a fronto-parietal network involved in a paired image discrimination task involving transitive inference. Some image pairs were derived from an implicit underlying sequence A>B>C>D>E, and some were independent (F>G, H>J, etc). Functional connectivity between the fronto-parietal regions and the default network regions depended on the presence of the underlying sequence relating the images. When subjects viewed learned and novel pairs from the sequence, connectivity between these two networks was higher than when subjects viewed learned and novel pairs from the independent sets.
Conclusions: These results suggest that default network regions were involved in maintaining the internal model that subserved discrimination of image pairs derived from the implicit sequence, and contributed to introspective access of an internal sequence model built during training. The default network may not be a unified entity with a specific function, but rather may interact with other functional networks in task-dependent ways.
Figures
Figure 1
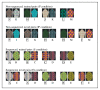
Cognitive task. Four tasks (P, IP, S, IS) were performed in 30-second blocks during fMRI scanning. Prior to scanning, subjects learned via feedback to discriminate the P and S pairs. During scanning, the subject had to indicate the correct stimulus in each pair via button press (no feedback was given). The pairs presented in the IP and IS conditions were novel and had to be inferred from the previously learned pairs in the P and S condition. For the sequenced set in S and IS, the underlying sequence A>B>C>D>E determined the correct response on all trained and novel pairs. There is no such relationship among pairs for the non-sequenced set in P and IP. Each image has an arbitrary letter label to assist in description; this was not shown to the subjects. For each pair, the correct response is indicated with an outlined letter.
Figure 2
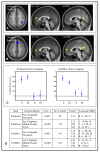
Fronto-parietal connectivity and sequence representation. The fronto-parietal network interacted more strongly with the default network during tasks that used an internal sequence representation. During scanning, participants responded to learned and novel pairs from a sequenced set and a non-sequenced set (Figure 1). Connectivity depended on the presence or absence of the underlying sequence. (A) Midline areas showed increased connectivity with the right parietal seed during sequence tasks. Functional connectivity was higher during S and IS conditions than P and IP, a positive Sequence by Parietal Seed psycho-physiological interaction. The orange/yellow colored voxels exhibited this interaction in a second-level analysis (p < 0.01). (B) Midline areas also showed increased connectivity with the preSMA seed during sequence tasks. Colored voxels exhibited a positive Sequence by PreSMA Seed psycho-physiological interaction (p < 0.01). (C) Connectivity between the fronto-parietal network and the posterior cingulate was high during sequence tasks S and IS, low during non-sequence tasks P and IP. Right: connectivity between right parietal seed and posterior cingulate from (A). Left: connectivity between PreSMA seed and posterior cingulate from (B). Error bars indicate the standard error of the mean. (D) The midline areas are within the default mode network. The table gives cluster coordinates in the MNI atlas space, corresponding to maps in (A) and (C). Voxels individually were p < 0.001 uncorrected, and reported clusters were significant at p < 0.01 corrected for multiple voxel comparisons based on cluster extent (one-tailed tests). The clusters were located in anterior cingulate, medial frontal gyrus, posterior cingulate, and precuneus, areas associated with the default network.
Figure 3
Fronto-parietal connectivity and inference. Some cortical regions showed stronger connectivity with the fronto-parietal seed ROIs during responses to learned pairs, versus to novel pairs. The table shows clusters of voxels with a significant Inference by Seed psycho-physiological interaction (p < 0.01 corrected), all of which showed higher connectivity with the corresponding seed ROI during the S and P conditions compared to IS and IP.
Figure 4
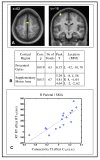
Connectivity/behavior correlations. Individual differences in connectivity between the right parietal seed region and the motor network partially explained the behavioral transitive inference effect. The behavioral TI effect was calculated from the reaction times for the four conditions: (RTIS-RTS)-(RTIP-RTP), which is the additional response time for the novel sequence (transitive inference) pairs relative to the learned sequence pairs, above and beyond the portion attributable to novelty only as determined from the non-sequence pairs. The connectivity TI effect was the parameter estimate for the sequence by inference by seed psycho-physiological interaction, analogous to (CIS-CS)-(CIP-CP) with C the connectivity between each voxel and the seed. (A) The behavioral and right parietal connectivity transitive inference effects were correlated in the bilateral supplementary motor area and left precentral gyrus. Significant positive correlation between the sequence by inference by seed psycho-physiological interaction and the behavioral TI effect was present in the colored voxels in a second-level analysis (p < 0.05). (B) The areas of correlation were the bilateral supplementary motor area and left precentral gyrus. The table gives cluster coordinates in the MNI atlas space corresponding to the map in (A). Voxels were p < 0.001 uncorrected, and reported clusters were significant at p < 0.05 corrected for multiple comparisons based on cluster extent (one-tailed tests). The clusters were located in areas associated with motor planning and execution. (C) The relationship between connectivity and behavioral TI effects was approximately linear. The plot shows the values from the SMA.
Similar articles
- Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses.
Sestieri C, Corbetta M, Romani GL, Shulman GL. Sestieri C, et al. J Neurosci. 2011 Mar 23;31(12):4407-20. doi: 10.1523/JNEUROSCI.3335-10.2011. J Neurosci. 2011. PMID: 21430142 Free PMC article. - Causal interactions between fronto-parietal central executive and default-mode networks in humans.
Chen AC, Oathes DJ, Chang C, Bradley T, Zhou ZW, Williams LM, Glover GH, Deisseroth K, Etkin A. Chen AC, et al. Proc Natl Acad Sci U S A. 2013 Dec 3;110(49):19944-9. doi: 10.1073/pnas.1311772110. Epub 2013 Nov 18. Proc Natl Acad Sci U S A. 2013. PMID: 24248372 Free PMC article. - Future planning: default network activity couples with frontoparietal control network and reward-processing regions during process and outcome simulations.
Gerlach KD, Spreng RN, Madore KP, Schacter DL. Gerlach KD, et al. Soc Cogn Affect Neurosci. 2014 Dec;9(12):1942-51. doi: 10.1093/scan/nsu001. Epub 2014 Feb 3. Soc Cogn Affect Neurosci. 2014. PMID: 24493844 Free PMC article. - Cooperation between the default mode network and the frontal-parietal network in the production of an internal train of thought.
Smallwood J, Brown K, Baird B, Schooler JW. Smallwood J, et al. Brain Res. 2012 Jan 5;1428:60-70. doi: 10.1016/j.brainres.2011.03.072. Epub 2011 Apr 3. Brain Res. 2012. PMID: 21466793 Review. - Brain connectivity and visual attention.
Parks EL, Madden DJ. Parks EL, et al. Brain Connect. 2013;3(4):317-38. doi: 10.1089/brain.2012.0139. Epub 2013 Jun 8. Brain Connect. 2013. PMID: 23597177 Free PMC article. Review.
Cited by
- Goal-congruent default network activity facilitates cognitive control.
Spreng RN, DuPre E, Selarka D, Garcia J, Gojkovic S, Mildner J, Luh WM, Turner GR. Spreng RN, et al. J Neurosci. 2014 Oct 15;34(42):14108-14. doi: 10.1523/JNEUROSCI.2815-14.2014. J Neurosci. 2014. PMID: 25319706 Free PMC article. - Noise concerns and post-processing procedures in cerebral blood flow (CBF) and cerebral blood volume (CBV) functional magnetic resonance imaging.
Donahue MJ, Juttukonda MR, Watchmaker JM. Donahue MJ, et al. Neuroimage. 2017 Jul 1;154:43-58. doi: 10.1016/j.neuroimage.2016.09.007. Epub 2016 Sep 11. Neuroimage. 2017. PMID: 27622397 Free PMC article. Review. - Connectivity in the human brain dissociates entropy and complexity of auditory inputs.
Nastase SA, Iacovella V, Davis B, Hasson U. Nastase SA, et al. Neuroimage. 2015 Mar;108:292-300. doi: 10.1016/j.neuroimage.2014.12.048. Epub 2014 Dec 20. Neuroimage. 2015. PMID: 25536493 Free PMC article. - The intrinsic connectivity distribution: a novel contrast measure reflecting voxel level functional connectivity.
Scheinost D, Benjamin J, Lacadie CM, Vohr B, Schneider KC, Ment LR, Papademetris X, Constable RT. Scheinost D, et al. Neuroimage. 2012 Sep;62(3):1510-9. doi: 10.1016/j.neuroimage.2012.05.073. Epub 2012 Jun 1. Neuroimage. 2012. PMID: 22659477 Free PMC article. - Potential use and challenges of functional connectivity mapping in intractable epilepsy.
Constable RT, Scheinost D, Finn ES, Shen X, Hampson M, Winstanley FS, Spencer DD, Papademetris X. Constable RT, et al. Front Neurol. 2013 May 22;4:39. doi: 10.3389/fneur.2013.00039. eCollection 2013. Front Neurol. 2013. PMID: 23734143 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources