Disulfiram treatment facilitates phosphoinositide 3-kinase inhibition in human breast cancer cells in vitro and in vivo - PubMed (original) (raw)
Disulfiram treatment facilitates phosphoinositide 3-kinase inhibition in human breast cancer cells in vitro and in vivo
Haijun Zhang et al. Cancer Res. 2010.
Abstract
Frequent genetic alterations of the components in the phosphoinositide 3-kinase (PI3K)/PTEN/AKT signaling pathway contribute greatly to breast cancer initiation and progression, which makes targeting this signaling pathway a promising therapeutic strategy for breast cancer treatment. In this study, we showed that in the presence of copper (Cu), disulfiram (DSF), a clinically used antialcoholism drug, could potently inhibit breast cancer cell growth regardless of the PIK3CA status. Surprisingly, the treatment with a mixture of DSF and copper (DSF-Cu) led to the decreased expression of PTEN protein and the activation of AKT in a dose- and time-dependent manner in different cell lines with or without PIK3CA mutations. Treatment of breast cancer cell lines with a combination of DSF-Cu and LY294002, a pan-PI3K inhibitor, resulted in the significant inhibition of cell growth when compared with either drug alone. In addition, the combined treatment of DSF and LY294002 significantly inhibited the growth of the breast tumor xenograft in nude mice induced by MDA-MB-231 cells expressing mutant PIK3CA-H1047R and PIK3CA-E545K, whereas neither DSF nor LY294002 alone could significantly retard tumor growth. Finally, the observed in vivo inhibitory effects are found associated with aberrant signaling alterations and apoptosis-inducing activities in tumor samples. Thus, our finding shows for the first time that treatment of breast cancer with DSF results in a novel feedback mechanism that activates AKT signaling. Our study also suggests that the combination of DSF and a PI3K inhibitor may offer a new combinational treatment model for breast cancer, particularly for those with PIK3CA mutations.
(c)2010 AACR.
Figures
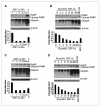
Figure 1
The effect of DSF-Cu on breast cancer cell lines with or without PIK3CA mutations. A and C, the effect of different doses of DSF-Cu (0–20 μm) on BT20 cells (A) and MDA-MB-231 cells (C) for 24 h. B and D, the effect of different time courses (0–48 h) of 10 μmol/L DSF treatment on BT20 cells (B) and MDA2-MB-231 cells (D). For all the figures, top, Western blot showing PARP cleavage; middle, the accumulation of ubiquitinated proteins (UB-PRS); bottom, cell viability after different time courses or doses for DSF-Cu treatment. The proteasome inhibitor MG132 is used as a positive control.
Figure 2
The effects of DSF-Cu on PTEN and AKT signaling in breast cancer cell lines with or without PIK3CA mutation. A, a Western blot showing the effects of different doses of DSF-Cu, CQ-Cu, and TM-Cu on PTEN protein expression in MDA-MB-231 cell. β-Actin was used as a protein loading control. B, kinetic effect of DSF-Cu (10 μmol/L) on PTEN and AKT signaling in MDA-MB-231 cells. MDA-MB-231 cells were treated with DMSO (DM), 10 μmol/L CuCl2 (Cu), DSF, or DSF-Cu mixture for the indicated number of hours. C and D, kinetic effects of high (10 μmol/L; C) versus low doses (0.5 μmol/L; D) of DSF-Cu on PTEN and AKT signaling in BT20 cell. BT20 cells were treated with DSF-Cu for the indicated number of hours. For B, C, and D, Western blots were performed with a PTEN, a p-AKT, and a total AKT antibody. β-Actin was used as a protein loading control.
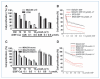
Figure 3
The mechanism of PTEN deregulation upon DSF-Cu treatment. A, the expression level of PTEN mRNA in BT20 cells after DSF-Cu treatment. Cells were treated with 10 μmol/L DSF-Cu for 0 to 10 h. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a RNA loading control. B and C, Western blot shows PTEN protein level after pretreatment with different protease inhibitor for 4 h, followed by DSF-Cu treatment for 6 h in BT20 cells (B) and MDA-MB-231 cells (C). D, caspase-3 inhibitor has no effect in preventing PTEN protein degradation upon DSF-Cu treatment. Different treatments were performed as indicated. Caspase-3 inhibitor (150 μmol/L) was pretreated for 4 h.
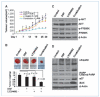
Figure 4
Combinational effects of DSF-Cu and LY294002 (LY) on breast cancer cell lines in vitro. A, the effect of combined treatment on BT20 and MDA-MB-231 cells. Three treatments were performed: (1) LY294002 at 10 μmol/L; (2) DSF-Cu at 0.05, 0.1, or 0.5 μmol/L; and (3) LY294002 plus DSF-Cu combination. DM, DMSO treatment as a negative control. B, scatter plot of cell proliferation (%) versus dose concentrations (μmol/L). Percent proliferations (%) were calculated as the ratios to the controls. Straight lines connect the mean cell proliferations. Synergism is indicated in both cell lines BT20 (P = 0.0014) and MDA-MB-231 (P = 0.0495) by ANOVA test. C, MDA-MB-231/vector, MDA-MB-231/PIK3CA-E545K, and MDA-MB-231/PIK3CA-H1047R cells were used to test the treatment regimens. Combined treatments (10 μmol/L LY294002 plus 1 μmol/L DSF) showed more significant inhibition of cell proliferation than a single treatment in all cell lines (all P < 0.002). D, the drug effects of combined treatment (10 μmol/L LY294002 plus DSF) among three cell lines are significant. The P values of one way ANOVA test at three concentration levels of DSF are 0.003, 0.019, and <0.001, respectively.
Figure 5
Combinational effects of DSF-Cu and LY294002 on the tumorigenesis of MDA-MB-231/PIK3CA-H1047R mutant cells in vivo. A, tumor growth chart showing the effect of the different treatments in vivo. Points, mean of tumor volume in each experimental group; bars, SD. B, comparison of final tumor weights after four different treatments in vivo. Top, representative pictures of one tumor group with four different treatments. Bottom, final weights after four treatments. Columns, mean; bars, SD. C, representative Western blot analysis of tumor tissue extract with antibodies against pAKT, AKT, p70S6K, pP70S6K, and β-actin. D, representative Western blots analysis of tumor tissue extract with antibodies against p27, BAX, PARP, ubiquitin, and β-actin.
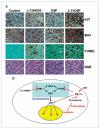
Figure 6
Alterations of apoptosis signaling in xenograft tumor samples and illustration of potential mechanism. A, IHC assays and TUNEL assay using mouse tumor samples. Tumors were collected after 29 d of treatment and the prepared tissue slides were analyzed with IHC using anti-p27 and anti-BAX antibodies, TUNEL assay, and H&E staining assay. P27 showed positive nucleus staining and BAX stained positively in the cytosol; arrows, stronger TUNEL-positive nuclei as well as apoptotic condensed nuclei. Top, the origins of all four-tumor samples. Magnification for all pictures, 10 × 40. B, illustration of a potential mechanism explaining the effects of current combinational treatment strategy, using the feedback loop induced by DSF-Cu treatment.
Similar articles
- Biomarkers of response to Akt inhibitor MK-2206 in breast cancer.
Sangai T, Akcakanat A, Chen H, Tarco E, Wu Y, Do KA, Miller TW, Arteaga CL, Mills GB, Gonzalez-Angulo AM, Meric-Bernstam F. Sangai T, et al. Clin Cancer Res. 2012 Oct 15;18(20):5816-28. doi: 10.1158/1078-0432.CCR-12-1141. Epub 2012 Aug 29. Clin Cancer Res. 2012. PMID: 22932669 Free PMC article. - Simultaneous inhibition of EGFR and PI3K enhances radiosensitivity in human breast cancer.
Li P, Zhang Q, Torossian A, Li ZB, Xu WC, Lu B, Fu S. Li P, et al. Int J Radiat Oncol Biol Phys. 2012 Jul 1;83(3):e391-7. doi: 10.1016/j.ijrobp.2011.12.090. Epub 2012 Mar 11. Int J Radiat Oncol Biol Phys. 2012. PMID: 22414288 - Targeting the PI3K/Akt/mTOR pathway with the pan-Akt inhibitor GDC-0068 in PIK3CA-mutant breast cancer brain metastases.
Ippen FM, Grosch JK, Subramanian M, Kuter BM, Liederer BM, Plise EG, Mora JL, Nayyar N, Schmidt SP, Giobbie-Hurder A, Martinez-Lage M, Carter SL, Cahill DP, Wakimoto H, Brastianos PK. Ippen FM, et al. Neuro Oncol. 2019 Nov 4;21(11):1401-1411. doi: 10.1093/neuonc/noz105. Neuro Oncol. 2019. PMID: 31173106 Free PMC article. - The favorable impact of PIK3CA mutations on survival: an analysis of 2587 patients with breast cancer.
Dumont AG, Dumont SN, Trent JC. Dumont AG, et al. Chin J Cancer. 2012 Jul;31(7):327-34. doi: 10.5732/cjc.012.10032. Epub 2012 May 24. Chin J Cancer. 2012. PMID: 22640628 Free PMC article. Review. - Efficacy of PI3K inhibitors in advanced breast cancer.
Verret B, Cortes J, Bachelot T, Andre F, Arnedos M. Verret B, et al. Ann Oncol. 2019 Dec 1;30(Suppl_10):x12-x20. doi: 10.1093/annonc/mdz381. Ann Oncol. 2019. PMID: 31859349 Free PMC article. Review.
Cited by
- A novel combination therapy for ER+ breast cancer suppresses drug resistance via an evolutionary double-bind.
Emond R, West J, Grolmusz V, Cosgrove P, Nath A, Anderson ARA, Bild AH. Emond R, et al. bioRxiv [Preprint]. 2024 Sep 6:2024.09.03.611032. doi: 10.1101/2024.09.03.611032. bioRxiv. 2024. PMID: 39282402 Free PMC article. Preprint. - Cuproptosis: Mechanisms, biological significance, and advances in disease treatment-A systematic review.
Pan C, Ji Z, Wang Q, Zhang Z, Wang Z, Li C, Lu S, Ge P. Pan C, et al. CNS Neurosci Ther. 2024 Sep;30(9):e70039. doi: 10.1111/cns.70039. CNS Neurosci Ther. 2024. PMID: 39267265 Free PMC article. Review. - Targeting cuproptosis for cancer therapy: mechanistic insights and clinical perspectives.
Zhang C, Huang T, Li L. Zhang C, et al. J Hematol Oncol. 2024 Aug 16;17(1):68. doi: 10.1186/s13045-024-01589-8. J Hematol Oncol. 2024. PMID: 39152464 Free PMC article. Review. - Pan-cancer analyses reveal molecular and clinical characteristics of cuproptosis regulators.
Wu C, Tan J, Wang X, Qin C, Long W, Pan Y, Li Y, Liu Q. Wu C, et al. Imeta. 2022 Dec 7;2(1):e68. doi: 10.1002/imt2.68. eCollection 2023 Feb. Imeta. 2022. PMID: 38868340 Free PMC article. - Revitalizing Cancer Treatment: Exploring the Role of Drug Repurposing.
Malla R, Viswanathan S, Makena S, Kapoor S, Verma D, Raju AA, Dunna M, Muniraj N. Malla R, et al. Cancers (Basel). 2024 Apr 11;16(8):1463. doi: 10.3390/cancers16081463. Cancers (Basel). 2024. PMID: 38672545 Free PMC article. Review.
References
- Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–9. - PubMed
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. - PubMed
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. - PubMed
- Ma YY, Wei SJ, Lin YC, et al. PIK3CA as an oncogene in cervical cancer. Oncogene. 2000;19:2739–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous