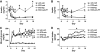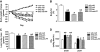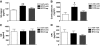Leptin deficiency causes insulin resistance induced by uncontrolled diabetes - PubMed (original) (raw)
. 2010 Jul;59(7):1626-34.
doi: 10.2337/db09-1918. Epub 2010 Apr 27.
Brent E Wisse, Joshua P Thaler, Shinsuke Oh-I, David A Sarruf, Kayoko Ogimoto, Karl J Kaiyala, Jonathan D Fischer, Miles E Matsen, Gerald J Taborsky Jr, Michael W Schwartz, Gregory J Morton
Affiliations
- PMID: 20424233
- PMCID: PMC2889761
- DOI: 10.2337/db09-1918
Leptin deficiency causes insulin resistance induced by uncontrolled diabetes
Jonathan P German et al. Diabetes. 2010 Jul.
Abstract
Objective: Depletion of body fat stores during uncontrolled, insulin-deficient diabetes (uDM) results in markedly reduced plasma leptin levels. This study investigated the role of leptin deficiency in the genesis of severe insulin resistance and related metabolic and neuroendocrine derangements induced by uDM.
Research design and methods: Adult male Wistar rats remained nondiabetic or were injected with the beta-cell toxin, streptozotocin (STZ) to induce uDM and subsequently underwent subcutaneous implantation of an osmotic minipump containing either vehicle or leptin at a dose (150 microg/kg/day) designed to replace leptin at nondiabetic plasma levels. To control for leptin effects on food intake, another group of STZ-injected animals were pair fed to the intake of those receiving leptin. Food intake, body weight, and blood glucose levels were measured daily, with body composition and indirect calorimetry performed on day 11, and an insulin tolerance test to measure insulin sensitivity performed on day 16. Plasma hormone and substrate levels, hepatic gluconeogenic gene expression, and measures of tissue insulin signal transduction were also measured.
Results: Physiologic leptin replacement prevented insulin resistance in uDM via a mechanism unrelated to changes in food intake or body weight. This effect was associated with reduced total body fat and hepatic triglyceride content, preservation of lean mass, and improved insulin signal transduction via the insulin receptor substrate-phosphatidylinositol-3-hydroxy kinase pathway in the liver, but not in skeletal muscle or adipose tissue. Although physiologic leptin replacement lowered blood glucose levels only slightly, it fully normalized elevated plasma glucagon and corticosterone levels and reversed the increased hepatic expression of gluconeogenic enzymes characteristic of rats with uDM.
Conclusions: We conclude that leptin deficiency plays a key role in the pathogenesis of severe insulin resistance and related endocrine disorders in uDM. Treatment of diabetes in humans may benefit from correction of leptin deficiency as well as insulin deficiency.
Figures
FIG. 1.
Physiologic leptin replacement attenuates diabetic hyperglycemia and diabetic hyperphagia in STZ-treated rats. Plasma insulin (A), plasma leptin (B), blood glucose (C), and mean daily food intake (D) in STZ-induced diabetic animals receiving either vehicle and fed ad libitum (□) or pair fed (♦), a physiologic replacement dose of leptin (♢) or nondiabetic controls (▲). *P < 0.05 vs. STZ-lep; #P < 0.05 vs. STZ-veh-PF.
FIG. 2.
Physiologic leptin replacement reduces fat mass and attenuates increased energy expenditure in STZ-treated rats. Body weight change (A), percent body fat mass (B), lean body mass (C), and _Vo_2 (D) measured using quantitative magnetic resonance and indirect calorimetry, respectively, in STZ-induced diabetic animals receiving either vehicle and fed ad libitum (□) or pair fed (♦), a physiologic replacement dose of leptin (♢) or nondiabetic controls (▲). *P < 0.05 vs. veh-veh; #P < 0.05 vs. STZ-lep. lbm, lean body mass.
FIG. 3.
Physiologic leptin replacement improves insulin sensitivity in STZ-treated rats. A: Blood glucose levels in STZ-induced diabetic animals on day 7 (▲) and 16 (□) after STZ-injection during an insulin tolerance test (2 units/kg). *P < 0.05 vs. day 7. Blood glucose levels (B), percent basal blood glucose levels (C), and the inverse integrated area under the percent basal glucose curve (D) in STZ-induced diabetic animals receiving either vehicle and fed ad libitum (□) or pair fed (♦), a physiologic replacement dose of leptin (♢) or nondiabetic controls (▲) during an ITT (2 units/kg) *P < 0.05 vs. STZ-lep; #P < 0.05 vs. STZ-veh-PF.
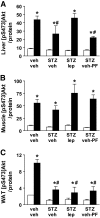
FIG. 4.
Physiologic leptin replacement increases hepatic insulin signal transduction. Effect of intraperitoneal (2 units/kg) insulin (■)-induced activation of serine phosphorylation of Akt compared with vehicle (□) in liver (A), muscle (tibialis anterior) (B), and WAT (epididymal fat) (C) in STZ-induced diabetic animals receiving either vehicle and fed ad libitum (STZ-veh) or pair fed (STZ-veh-PF) a physiologic replacement dose of leptin (STZ-lep) or nondiabetic controls (veh-veh). *P < 0.05 vs. veh-veh-veh; #P < 0.05 vs. veh-veh-ins.
FIG. 5.
Physiologic leptin replacement reduces hyperglucagonemia and hypercorticosteronemia. Arterial plasma glucagon (A), corticosterone (B), norepinephrine (NE) (C), and epinephrine (EPI) (D) levels in nondiabetic controls (veh-veh) or in STZ-induced diabetic animals receiving either vehicle (STZ-veh) or a physiologic replacement dose of leptin (STZ-lep). *P < 0.05 vs. veh-veh; #P < 0.05 vs. STZ-lep.
FIG. 6.
Physiologic leptin replacement reduces plasma and hepatic lipid content and gluconeogenic gene expression. Plasma NEFAs obtained from tail-vein samples (A), hepatic triglyceride content (B) and expression of G6Pase (C), Pepck (D), _Pgc-1_α (E), and Igfbp2 (F) using real-time PCR in nondiabetic controls (veh-veh) or in STZ-induced diabetic animals receiving either vehicle and fed ad libitum (STZ-veh) or pair fed (STZ-veh-PF) or a physiologic replacement dose of leptin (STZ-lep). *P < 0.05 vs. veh-veh; #P < 0.05 vs. STZ-lep.
Similar articles
- Partial leptin deficiency confers resistance to diet-induced obesity in mice.
Zhao S, Li N, Zhu Y, Straub L, Zhang Z, Wang MY, Zhu Q, Kusminski CM, Elmquist JK, Scherer PE. Zhao S, et al. Mol Metab. 2020 Jul;37:100995. doi: 10.1016/j.molmet.2020.100995. Epub 2020 Apr 11. Mol Metab. 2020. PMID: 32289482 Free PMC article. - Leptin therapy reverses hyperglycemia in mice with streptozotocin-induced diabetes, independent of hepatic leptin signaling.
Denroche HC, Levi J, Wideman RD, Sequeira RM, Huynh FK, Covey SD, Kieffer TJ. Denroche HC, et al. Diabetes. 2011 May;60(5):1414-23. doi: 10.2337/db10-0958. Epub 2011 Apr 4. Diabetes. 2011. PMID: 21464443 Free PMC article. - Intracerebroventricular leptin infusion improves glucose homeostasis in lean type 2 diabetic MKR mice via hepatic vagal and non-vagal mechanisms.
Li X, Wu X, Camacho R, Schwartz GJ, LeRoith D. Li X, et al. PLoS One. 2011 Feb 17;6(2):e17058. doi: 10.1371/journal.pone.0017058. PLoS One. 2011. PMID: 21379576 Free PMC article. - Analysis of paradoxical observations on the association between leptin and insulin resistance.
Ceddia RB, Koistinen HA, Zierath JR, Sweeney G. Ceddia RB, et al. FASEB J. 2002 Aug;16(10):1163-76. doi: 10.1096/fj.02-0158rev. FASEB J. 2002. PMID: 12153984 Review. - The glucoregulatory actions of leptin.
D'souza AM, Neumann UH, Glavas MM, Kieffer TJ. D'souza AM, et al. Mol Metab. 2017 May 4;6(9):1052-1065. doi: 10.1016/j.molmet.2017.04.011. eCollection 2017 Sep. Mol Metab. 2017. PMID: 28951828 Free PMC article. Review.
Cited by
- Gut-brain mechanisms controlling glucose homeostasis.
Scarlett JM, Schwartz MW. Scarlett JM, et al. F1000Prime Rep. 2015 Jan 5;7:12. doi: 10.12703/P7-12. eCollection 2015. F1000Prime Rep. 2015. PMID: 25705395 Free PMC article. Review. - Relation of leptin, ghrelin and inflammatory cytokines with body mass index in pulmonary tuberculosis patients with and without type 2 diabetes mellitus.
Zheng Y, Ma A, Wang Q, Han X, Cai J, Schouten EG, Kok FJ, Li Y. Zheng Y, et al. PLoS One. 2013 Nov 8;8(11):e80122. doi: 10.1371/journal.pone.0080122. eCollection 2013. PLoS One. 2013. PMID: 24260344 Free PMC article. - How Should We Think About the Role of the Brain in Glucose Homeostasis and Diabetes?
Deem JD, Muta K, Scarlett JM, Morton GJ, Schwartz MW. Deem JD, et al. Diabetes. 2017 Jul;66(7):1758-1765. doi: 10.2337/dbi16-0067. Epub 2017 Jun 11. Diabetes. 2017. PMID: 28603139 Free PMC article. No abstract available. - Leptin therapy, insulin sensitivity, and glucose homeostasis.
Paz-Filho G, Mastronardi C, Wong ML, Licinio J. Paz-Filho G, et al. Indian J Endocrinol Metab. 2012 Dec;16(Suppl 3):S549-55. doi: 10.4103/2230-8210.105571. Indian J Endocrinol Metab. 2012. PMID: 23565489 Free PMC article. - Glucose Variability: Timing, Risk Analysis, and Relationship to Hypoglycemia in Diabetes.
Kovatchev B, Cobelli C. Kovatchev B, et al. Diabetes Care. 2016 Apr;39(4):502-10. doi: 10.2337/dc15-2035. Diabetes Care. 2016. PMID: 27208366 Free PMC article.
References
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM: Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–432 - PubMed
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P: Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 1995;269:546–549 - PubMed
- Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte D, Jr, Woods SC, Seeley RJ, Weigle DS: Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes 1996;45:531–535 - PubMed
- Chinookoswong N, Wang JL, Shi ZQ: Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes 1999;48:1487–1492 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK074758/DK/NIDDK NIH HHS/United States
- DK-083042/DK/NIDDK NIH HHS/United States
- DK-035816/DK/NIDDK NIH HHS/United States
- R01 DK050154/DK/NIDDK NIH HHS/United States
- DK-050154-13/DK/NIDDK NIH HHS/United States
- P30 DK035816/DK/NIDDK NIH HHS/United States
- R01 DK052989/DK/NIDDK NIH HHS/United States
- DK-052989/DK/NIDDK NIH HHS/United States
- R01 DK083042/DK/NIDDK NIH HHS/United States
- R56 DK050154/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical