Proteolysis of Rad17 by Cdh1/APC regulates checkpoint termination and recovery from genotoxic stress - PubMed (original) (raw)
Proteolysis of Rad17 by Cdh1/APC regulates checkpoint termination and recovery from genotoxic stress
Liyong Zhang et al. EMBO J. 2010.
Abstract
Recent studies have shown a critical function for the ubiquitin-proteasome system (UPS) in regulating the signalling network for DNA damage responses and DNA repair. To search for new UPS targets in the DNA damage signalling pathway, we have carried out a non-biased assay to identify fast-turnover proteins induced by various types of genotoxic stress. This endeavour led to the identification of Rad17 as a protein exhibiting a distinctive pattern of upregulation followed by subsequent degradation after exposure to UV radiation in human primary cells. Our characterization showed that UV-induced Rad17 oscillation is mediated by Cdh1/APC, a ubiquitin-protein ligase. Studies using a degradation-resistant Rad17 mutant demonstrated that Rad17 stabilization prevents the termination of checkpoint signalling, which in turn attenuates the cellular re-entry into cell-cycle progression. The findings provide an insight into how the proteolysis of Rad17 by Cdh1/APC regulates the termination of checkpoint signalling and the recovery from genotoxic stress.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
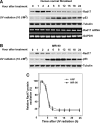
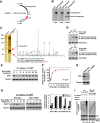
Time-dependent Rad17 proteolysis in response to UV in human primary cells. (A) On DNA damage, Rad17 accumulates, reaching a plateau in 1 h in HNF cells. Rad17 protein levels then gradually decline after 4 h and reach a basal level after 6 to 8 h. The UV-activated damage response was indicated by the kinetics of p53. The kinetics of Rad17 mRNA after exposure of cells to UV was examined using RT–PCR, with no significant alteration observed in Rad17 mRNA levels. Equivalent amount of loaded protein was estimated by measuring tubulin. (B) A similar pattern of Rad17 alteration induced by UV was found in another human primary cell type, IMR-90. (C) Summary of UV-induced alterations of Rad17 protein levels in HNF and IMR-90 cells.
Figure 2
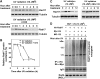
UV-induced Rad17 degradation is through a ubiquitin-proteasome pathway. (A) Alteration of Rad17 protein half-life in response to UV radiation. HNF cells were pretreated for 15 min with 20 mM cycloheximide followed by UV treatment. Rad17 protein levels drop to basal levels in 8 h after exposure to UV in the absence of cycloheximide, whereas UV exposure decreased the apparent half-life of the Rad17 protein from ∼8 to 4 h in the presence of cycloheximide. (B) Summary of UV-induced Rad17 degradation in the absence or presence of cycloheximide. (C) Pre-incubation of proteasome inhibitor MG-132 (10 uM) attenuates the UV-induced Rad17 downregulation in HNF cells. (D) Rad17 is ubiquitylated responding to UV radiation. Myc-tagged ubiquitin and HA-tagged Rad17 were co-transfected into HNF cells. Cells were exposed to UV and harvested at the indicated time points. HA-Rad17 was pulled down by antibody against HA. The ubiquitin-conjugated HA-Rad17 was subsequently detected by immunoblotting using anti-Myc antibody.
Figure 3
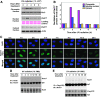
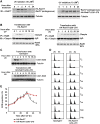
Proteolytic regulation of Rad17 is associated with chromatin-based DNA damage response and dissociation of checkpoint complex. (A) UV-induced Rad17 translocation and its degradation on chromatin. HNF cells were treated with UV and harvested at indicated time points. Cell pellets were fractionated into cytosol, soluble nuclear and chromatin-enriched portions. UV-induced Rad17 degradation was examined for different cellular compartments. Normalized protein loading for chromatin, nuclear and cytosol fractions was measured by ORC2, Ponceau S staining and actin, respectively. (B) Summary of UV-induced Rad17 alterations in different cellular compartment. (C) Transient formation of Rad17 nuclear foci in response to UV radiation. HNF cells were treated with UV and fixed at the different time points followed by immunostaining with antibody against Rad17. (D) Kinetics of interaction of Rad17 and 9-1-1 induced by DNA damage is regulated by Rad17 proteolysis. HNF cells were exposed to UV and collected at the indicated time points. Endogenous Rad9 complexes were immunoprecipitated with anti-Rad9 antibody (polyclonal). Rad9 IP complex was then immunoblotted with antibody against Rad17. Co-IP Rad9 was determined by immunoblotting using antibody (monoclonal) against Rad9. (E) Kinetics of interaction of Rad17 and ATR in response to DNA damage. Flag-tagged ATR was transfected into HNF cells. Ectopically expressed Flag-ATR was immunoprecipitated with anti-Flag antibody. ATR IP complexes were immunoblotted with Rad17 antibody. Co-IP Flag-ATR was measured by immunoblotting using antibody against Flag.
Figure 4
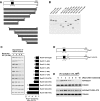
Mapping of the functional domain that mediates UV-induced Rad17 degradation. (A) Schematic presentation of Rad17 structure and strategy for mutagenesis. (B) Expression of a set of Rad17 mutants. (C) Stability of Rad17 mutants in response to UV radiation. A series of HA-tagged Rad17 mutants were transfected into HNF cells. Stability for each mutant was measured by immunoblotting. (D) Design of Rad17 stable mutant based on information of protein degradation summarized in (C). Amino acids from 230 to 270 were deleted for a stable Rad17 mutant. (E) Both HA-tagged wild type and stable Rad17 were transfected into HNF cells and their stability in response to UV radiation was evaluated by immunoblotting.
Figure 5
Identification of Cdh1/APC as a putative E3 ligase that mediates UV-induced Rad17 degradation. (A) Engineered Rad17-TAP construct. (B) Verification of engineered Rad17-TAP expression. Stably expressed and HA and Flag-tagged Rad17 was examined by antibodies against HA and Flag. (C) A silver-stained protein profile of purified Rad17 complexes in response to UV. Analyses of mass spectrometry indicate the presence of Cdh1 in the Rad17 complex. The IgG-agroase was used as control resin for the purification. Analyses of mass spectrometry indicate the presence of Cdh1 in the Rad17 complex. (D) Verification of interaction between Rad17 and Cdh1 by co-immunoprecipitation. Reversed immunoprecipitation coupled western blotting were carried out by using polyclonal anti-Rad17 and monoclonal anti-Cdh1. (E) Measurement of alteration in Cdh1 protein levels at various time points after exposure of cell to UV radiation. (F) Summary of (E). (G) Depletion of Cdh1 in HNF cells by RNA interference. (H) Effect of Cdh1 depletion on UV-induced Rad17 proteolysis. Knockdown of Cdh1 attenuates the UV-mediated Rad17 proteolysis. (I) Depletion of Cdh1 leads to attenuation of UV-induced Rad17 ubiquitylation. Cells were transfected with plasmid as indicated and were harvested 5 h after exposure to UV. HA-tagged Rad17 was pulled down by antibody against HA. The ubiquitin-conjugated HA-Rad17 was then detected by immunoblotting using anti-Myc antibody.
Figure 6
Stabilization of Rad17 results in a prolonged interaction between Rad17 and checkpoint complex. (A, B) Measurement of kinetics of interacted wild type and non-degradable Rad17 with checkpoint complex after exposure of cell to UV radiation. A HA-tagged Rad17 stable mutant as well as a control (HA-Rad17) were transfected into HNF cells. Association of the Rad17 stable mutant as well as HA-Rad17 with 9-1-1 complexes in response to UV was detected by immunoprecipitation using antibody against Rad9 followed by immunoblotting with anti-HA antibody. Total expression of Rad9 was determined with 1/12th of the volume of the total cellular extract used for immunoprecipitation. (C) Both wild type and stable Rad17 were transfected into HNF cells. Transfected cells were subsequently exposed to UV and harvested at indicated time points. Protein stability for wild type and stable Rad17 were measured by immunoblotting using anti-HA antibody based on chromatin fraction. Equal protein loading was estimated by measuring Orc2. (D) Summary of UV-induced protein turnover for wild type and stable Rad17.
Figure 7
Stabilized Rad17 disrupts termination of checkpoint signalling, which in turn impairs re-entry into cell-cycle progression. (A) Expression of stabilized Rad17 interferes the UV-induced proteolysis of endogenous Rad17, which in turn reduces the turnover rate of endogenous Rad17 after exposure to UV. (B) Stabilization of Rad17 enhances a prolonged association between Claspin and checkpoint complex as demonstrated by co-IP assay. (C) Stabilized Rad17 results in Chk1 being phosphorylated for an expanded time. (D) Stabilization of Rad17 is associated with delayed re-entry into cell-cycle progression after exposure to UV radiation. U2OS cells were transfected with wild type and stable Rad17 and then were treated with UV radiation (10 J/M2). Cells were collected at the indicated time points and stained with propidium iodide for FACS analyses. UV radiation causes a delay at G2/M. UV-induced G2/M delay in cells transfected with wild-type Rad17 gradually recovered after 30 h of exposure to UV, whereas transfection of Rad17 stable mutant significantly attenuated the time-dependent recovery from G2/M delay. (E) Summary for experiment in (D).
Figure 8
A proposed model for the function of Rad17 proteolytic regulation in regulating UV-induced checkpoint response.
Similar articles
- Regulation of Rad17 protein turnover unveils an impact of Rad17-APC cascade in breast carcinogenesis and treatment.
Zhou Z, Jing C, Zhang L, Takeo F, Kim H, Huang Y, Liu Z, Wan Y. Zhou Z, et al. J Biol Chem. 2013 Jun 21;288(25):18134-45. doi: 10.1074/jbc.M113.456962. Epub 2013 May 1. J Biol Chem. 2013. PMID: 23637229 Free PMC article. - ATR-Chk1-APC/CCdh1-dependent stabilization of Cdc7-ASK (Dbf4) kinase is required for DNA lesion bypass under replication stress.
Yamada M, Watanabe K, Mistrik M, Vesela E, Protivankova I, Mailand N, Lee M, Masai H, Lukas J, Bartek J. Yamada M, et al. Genes Dev. 2013 Nov 15;27(22):2459-72. doi: 10.1101/gad.224568.113. Genes Dev. 2013. PMID: 24240236 Free PMC article. - ATR/ATM-mediated phosphorylation of human Rad17 is required for genotoxic stress responses.
Bao S, Tibbetts RS, Brumbaugh KM, Fang Y, Richardson DA, Ali A, Chen SM, Abraham RT, Wang XF. Bao S, et al. Nature. 2001 Jun 21;411(6840):969-74. doi: 10.1038/35082110. Nature. 2001. PMID: 11418864 - Ku, Artemis, and ataxia-telangiectasia-mutated: signalling networks in DNA damage.
Morio T, Kim H. Morio T, et al. Int J Biochem Cell Biol. 2008;40(4):598-603. doi: 10.1016/j.biocel.2007.12.007. Epub 2007 Dec 24. Int J Biochem Cell Biol. 2008. PMID: 18243767 Review. - DNA repair: how to PIKK a partner.
Hiom K. Hiom K. Curr Biol. 2005 Jun 21;15(12):R473-5. doi: 10.1016/j.cub.2005.06.012. Curr Biol. 2005. PMID: 15964271 Review.
Cited by
- Cdc14A and Cdc14B Redundantly Regulate DNA Double-Strand Break Repair.
Lin H, Ha K, Lu G, Fang X, Cheng R, Zuo Q, Zhang P. Lin H, et al. Mol Cell Biol. 2015 Nov;35(21):3657-68. doi: 10.1128/MCB.00233-15. Epub 2015 Aug 17. Mol Cell Biol. 2015. PMID: 26283732 Free PMC article. - The APC/C Ubiquitin Ligase: From Cell Biology to Tumorigenesis.
Penas C, Ramachandran V, Ayad NG. Penas C, et al. Front Oncol. 2012 Jan 9;1:60. doi: 10.3389/fonc.2011.00060. eCollection 2011. Front Oncol. 2012. PMID: 22655255 Free PMC article. - Interplay between arginine methylation and ubiquitylation regulates KLF4-mediated genome stability and carcinogenesis.
Hu D, Gur M, Zhou Z, Gamper A, Hung MC, Fujita N, Lan L, Bahar I, Wan Y. Hu D, et al. Nat Commun. 2015 Sep 30;6:8419. doi: 10.1038/ncomms9419. Nat Commun. 2015. PMID: 26420673 Free PMC article. - The Anaphase-Promoting Complex/Cyclosome Is a Cellular Ageing Regulator.
Hu X, Jin X, Cao X, Liu B. Hu X, et al. Int J Mol Sci. 2022 Dec 5;23(23):15327. doi: 10.3390/ijms232315327. Int J Mol Sci. 2022. PMID: 36499653 Free PMC article. Review. - Regulation of XIAP Turnover Reveals a Role for USP11 in Promotion of Tumorigenesis.
Zhou Z, Luo A, Shrivastava I, He M, Huang Y, Bahar I, Liu Z, Wan Y. Zhou Z, et al. EBioMedicine. 2017 Feb;15:48-61. doi: 10.1016/j.ebiom.2016.12.014. Epub 2016 Dec 23. EBioMedicine. 2017. PMID: 28040451 Free PMC article.
References
- Abraham RT (2001) Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev 15: 2177–2196 - PubMed
- Bao S, Tibbetts RS, Brumbaugh KM, Fang Y, Richardson DA, Ali A, Chen SM, Abraham RT, Wang XF (2001) ATR/ATM-mediated phosphorylation of human Rad17 is required for genotoxic stress responses. Nature 411: 969–974 - PubMed
- Budzowska M, Jaspers I, Essers J, de Waard H, van Drunen E, Hanada K, Beverloo B, Hendriks RW, de Klein A, Kanaar R, Hoeijmakers JH, Maas A (2004) Mutation of the mouse Rad17 gene leads to embryonic lethality and reveals a role in DNA damage-dependent recombination. EMBO J 23: 3548–3558 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous