Multifactorial anticancer effects of digalloyl-resveratrol encompass apoptosis, cell-cycle arrest, and inhibition of lymphendothelial gap formation in vitro - PubMed (original) (raw)
. 2010 Apr 27;102(9):1361-70.
doi: 10.1038/sj.bjc.6605656.
P Saiko, C Vonach, K Viola, N Huttary, N Stark, R Popescu, M Gridling, N T-P Vo, I Herbacek, A Davidovits, B Giessrigl, S Venkateswarlu, S Geleff, W Jäger, M Grusch, D Kerjaschki, W Mikulits, T Golakoti, M Fritzer-Szekeres, T Szekeres, G Krupitza
Affiliations
- PMID: 20424615
- PMCID: PMC2865764
- DOI: 10.1038/sj.bjc.6605656
Multifactorial anticancer effects of digalloyl-resveratrol encompass apoptosis, cell-cycle arrest, and inhibition of lymphendothelial gap formation in vitro
S Madlener et al. Br J Cancer. 2010.
Abstract
Background: Digalloyl-resveratrol (di-GA) is a synthetic compound aimed to combine the biological effects of the plant polyhydroxy phenols gallic acid and resveratrol, which are both radical scavengers and cyclooxygenase inhibitors exhibiting anticancer activity. Their broad spectrum of activities may probably be due to adjacent free hydroxyl groups.
Methods: Protein activation and expression were analysed by western blotting, deoxyribonucleoside triphosphate levels by HPLC, ribonucleotide reductase activity by (14)C-cytidine incorporation into nascent DNA and cell-cycle distribution by FACS. Apoptosis was measured by Hoechst 33258/propidium iodide double staining of nuclear chromatin and the formation of gaps into the lymphendothelial barrier in a three-dimensional co-culture model consisting of MCF-7 tumour cell spheroids and human lymphendothelial monolayers.
Results: In HL-60 leukaemia cells, di-GA activated caspase 3 and dose-dependently induced apoptosis. It further inhibited cell-cycle progression in the G1 phase by four different mechanisms: rapid downregulation of cyclin D1, induction of Chk2 with simultaneous downregulation of Cdc25A, induction of the Cdk-inhibitor p21(Cip/Waf) and inhibition of ribonucleotide reductase activity resulting in reduced dCTP and dTTP levels. Furthermore, di-GA inhibited the generation of lymphendothelial gaps by cancer cell spheroid-secreted lipoxygenase metabolites. Lymphendothelial gaps, adjacent to tumour bulks, can be considered as gates facilitating metastatic spread.
Conclusion: These data show that di-GA exhibits three distinct anticancer activities: induction of apoptosis, cell-cycle arrest and disruption of cancer cell-induced lymphendothelial disintegration.
Figures
Figure 1
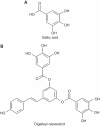
Chemical structures of (A) gallic acid (GA) and (B) digalloyl-resveratrol (di-GA).
Figure 2
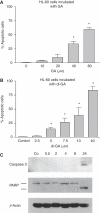
Induction of apoptosis by (A) GA and (B) di-GA in HL-60 cells. Cells were incubated with increasing concentrations of drugs for 24 h, and then double stained with Hoechst 33258 and propidium iodide. Afterwards cells were examined under the microscope with UV light connected to a DAPI filter. Nuclei with a morphological phenotype indicating apoptosis were counted and percentages of apoptotic cells were calculated. Experiments were conducted in triplicate. Error bars indicate s.e.m., asterisks significance (P<0.05). (C) Activation of caspase 3 and cleavage of PARP on treatment with di-GA. Logarithmically growing HL-60 cells were incubated with 10 μ
M
di-GA for 0.5, 2, 4, 8 and 24 h. Afterwards cells were lysed and protein expression was analysed by western blotting. The anti-caspase 3 antibody recognises only the cleaved peptide indicating its activation. Anti-PARP antibody recognises the full-length form (116 kDa) and the signature-type cleaved product (85 kDa) that is generated by active caspase 3. The antibody against _β_-actin was used to monitor equal sample loading.
Figure 3
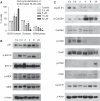
Effect of di-GA on the cell cycle of HL-60 cells. (A) Logarithmically growing HL-60 cells were incubated with increasing concentrations of di-GA for 24 h and then subjected to FACS analysis. Experiments were conducted in triplicate. Error bars indicate s.e.m., asterisks significance (P<0.05). HL-60 cells were incubated with 10 μ
M
di-GA for 0.5, 2, 4, 8 and 24 h, lysed, and the (B) expression of p21Cip/Waf, the phosphorylation of threonine202/tyrosine204-Erk1/2 (p-Erk1/2) and serine217/221-MEK1/2 (p-MEK), and (C) phosphorylation of threonine68-Chk2 (p-Chk2), serine17-Cdc25A (p-Cdc25A), tyrosine15-Cdc2 (p-Cdc2), and the protein levels of cyclin D1, E were analysed by western blotting. _β_-Actin served as loading control.
Figure 4
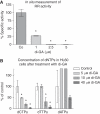
(A) Measurement of the in situ effect of di-GA on ribonucleotide reductase (RR) activity. HL-60 cells were incubated with 1, 2.5 and 5 μ
M
di-GA for 24 h at 37 °C in a humidified atmosphere containing 5% CO2 to assess changes in RR in situ activity. Then, cells were pulsed with 14C-cytidine (Sigma-Aldrich; 3 _μ_l in a 5 ml cell suspension) for 30 min at 37 °C. Afterwards the cells were collected and the radioactivity that became incorporated into genomic DNA was measured. (B) Effect of di-GA on intracellular dNTP pools in HL-60 cells. HL-60 cells were incubated with 5, 10 and 40 μ
M
di-GA for 24 h. Then the cells were prepared for HPLC analysis and the dNTP levels were determined according to the protocol described in the ‘Materials and methods’ section. Experiments were conducted in triplicate. Error bars indicate s.e.m., asterisks significance (P<0.05).
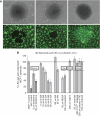
Figure 5
Effect of di-GA on MCF-7 spheroid-induced gap formation in lymphendothelial cell monolayers. (A) LEC monolayers that were exposed to MCF-7 spheroid (left side panels), MCF-7 spheroid treated with 40 μ
M
di-GA (middle panels) and HLF spheroid (right side panels). Upper panels are phase-contrast micrographs showing the respective spheroids, the panels below show the identical power fields using FITC filter and exhibit green stained LECs underneath the respective spheroids. Bars in the lower right corners of upper panels indicate 100 μ
M
. (B) MCF-7 tumor spheroids were preincubated with solvent (control), or 5 and 50 μ
M
berberine; 5, 10, 25, 40 and 80 μ
M
di-GA; 80 μ
M
GA; 50 μ
M
RV; 50 μ
M
NDGA; 100 μ
M
baicalein; 200 μ
M
aspirin; 25 μ
M
mannitol; 600 U ml−1 catalase; 200 μ
M
carboxy-PTIO and 100 μ
M
probucol, and then placed on top of cytotracker stained LEC monolayers that were also treated with respective agents for 4 h. Then, the size of the gaps that were formed in the LEC monolayers by MCF-7 spheroids (through repulsion of LECs) was measured using an inverted microscope connected to an FITC filter and equipped with Axiovision 4.5 software (Carl Zeiss). As negative controls normal human lung fibroblast (HLF) spheroids were used. Rho/rac (small GTPases regulating cell migration), LOX (lipoxygenase), COX (cyclooxygenases) and ROS (reactive oxygen species) indicate which mechanisms and phenomena are inhibited by the respective agents. Experiments were conducted in triplicate, and the underneath areas of at least 12 spheroids were analysed. Error bars indicate s.e.m., asterisks significance (P<0.05).
Similar articles
- Digalloylresveratrol, a novel resveratrol analog inhibits the growth of human pancreatic cancer cells.
Saiko P, Graser G, Giessrigl B, Steinmann MT, Schuster H, Lackner A, Grusch M, Krupitza G, Jaeger W, Somepalli V, Golakoti T, Fritzer-Szekeres M, Szekeres T. Saiko P, et al. Invest New Drugs. 2013 Oct;31(5):1115-24. doi: 10.1007/s10637-013-0009-x. Epub 2013 Aug 14. Invest New Drugs. 2013. PMID: 23943154 - Novel resveratrol analogs induce apoptosis and cause cell cycle arrest in HT29 human colon cancer cells: inhibition of ribonucleotide reductase activity.
Saiko P, Pemberger M, Horvath Z, Savinc I, Grusch M, Handler N, Erker T, Jaeger W, Fritzer-Szekeres M, Szekeres T. Saiko P, et al. Oncol Rep. 2008 Jun;19(6):1621-6. Oncol Rep. 2008. PMID: 18497974 - Digalloylresveratrol, a new phenolic acid derivative induces apoptosis and cell cycle arrest in human HT-29 colon cancer cells.
Bernhaus A, Fritzer-Szekeres M, Grusch M, Saiko P, Krupitza G, Venkateswarlu S, Trimurtulu G, Jaeger W, Szekeres T. Bernhaus A, et al. Cancer Lett. 2009 Feb 18;274(2):299-304. doi: 10.1016/j.canlet.2008.09.020. Epub 2008 Oct 25. Cancer Lett. 2009. PMID: 18952370 - N-hydroxy-N'-(3,4,5-trimethoxyphenyl)-3,4,5-trimethoxy-benzamidine, a novel resveratrol analog, inhibits ribonucleotide reductase in HL-60 human promyelocytic leukemia cells: synergistic antitumor activity with arabinofuranosylcytosine.
Saiko P, Ozsvar-Kozma M, Bernhaus A, Jaschke M, Graser G, Lackner A, Grusch M, Horvath Z, Madlener S, Krupitza G, Handler N, Erker T, Jaeger W, Fritzer-Szekeres M, Szekeres T. Saiko P, et al. Int J Oncol. 2007 Nov;31(5):1261-6. Int J Oncol. 2007. PMID: 17912455 - Gallic Acid: A Potential Anti-Cancer Agent.
Jiang Y, Pei J, Zheng Y, Miao YJ, Duan BZ, Huang LF. Jiang Y, et al. Chin J Integr Med. 2022 Jul;28(7):661-671. doi: 10.1007/s11655-021-3345-2. Epub 2021 Nov 10. Chin J Integr Med. 2022. PMID: 34755289 Review.
Cited by
- Flavonoids Distinctly Stabilize Lymph Endothelial- or Blood Endothelial Disintegration Induced by Colon Cancer Spheroids SW620.
Berenda J, Smöch C, Stadlbauer C, Mittermair E, Taxauer K, Huttary N, Krupitza G, Krenn L. Berenda J, et al. Molecules. 2020 Apr 29;25(9):2066. doi: 10.3390/molecules25092066. Molecules. 2020. PMID: 32365473 Free PMC article. - Chemical Composition of Scrophularia lucida and the Effects on Tumor Invasiveness in Vitro.
Lewenhofer V, Schweighofer L, Ledermüller T, Eichsteininger J, Kählig H, Zehl M, Nguyen CH, Krupitza G, Özmen A, Krenn L. Lewenhofer V, et al. Front Pharmacol. 2018 Apr 3;9:304. doi: 10.3389/fphar.2018.00304. eCollection 2018. Front Pharmacol. 2018. PMID: 29666580 Free PMC article. - A robust in vitro model for trans-lymphatic endothelial migration.
Xiong Y, Brinkman CC, Famulski KS, Mongodin EF, Lord CJ, Hippen KL, Blazar BR, Bromberg JS. Xiong Y, et al. Sci Rep. 2017 May 9;7(1):1633. doi: 10.1038/s41598-017-01575-w. Sci Rep. 2017. PMID: 28487567 Free PMC article. - Colon cancer cell-derived 12(S)-HETE induces the retraction of cancer-associated fibroblast via MLC2, RHO/ROCK and Ca2+ signalling.
Stadler S, Nguyen CH, Schachner H, Milovanovic D, Holzner S, Brenner S, Eichsteininger J, Stadler M, Senfter D, Krenn L, Schmidt WM, Huttary N, Krieger S, Koperek O, Bago-Horvath Z, Brendel KA, Marian B, de Wever O, Mader RM, Giessrigl B, Jäger W, Dolznig H, Krupitza G. Stadler S, et al. Cell Mol Life Sci. 2017 May;74(10):1907-1921. doi: 10.1007/s00018-016-2441-5. Epub 2016 Dec 24. Cell Mol Life Sci. 2017. PMID: 28013338 Free PMC article. - NF-κB contributes to MMP1 expression in breast cancer spheroids causing paracrine PAR1 activation and disintegrations in the lymph endothelial barrier in vitro.
Nguyen CH, Senfter D, Basilio J, Holzner S, Stadler S, Krieger S, Huttary N, Milovanovic D, Viola K, Simonitsch-Klupp I, Jäger W, de Martin R, Krupitza G. Nguyen CH, et al. Oncotarget. 2015 Nov 17;6(36):39262-75. doi: 10.18632/oncotarget.5741. Oncotarget. 2015. PMID: 26513020 Free PMC article.
References
- Agarwal C, Tyagi A, Agarwal R (2006) Gallic acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via ATM-Chk2 activation, leading to cell cycle arrest, and induces apoptosis in human prostate carcinoma DU145 cells. Mol Cancer Ther 5(12): 3294–3302 - PubMed
- Alitalo K, Tammela T, Petrova TV (2005) Lymphangiogenesis in development and human disease. Nature 438: 946–953 - PubMed
- Ata N, Oku T, Hattori M, Fujii H, Nakajima M, Saiki I (1996) Inhibition by galloylglucose (GG6-10) of tumor invasion through extracellular matrix and gelatinase-mediated degradation of type IV collagens by metastatic tumor cells. Oncol Res 8(12): 503–511 - PubMed
- Bernhaus A, Fritzer-Szekeres M, Grusch M, Saiko P, Krupitza G, Venkateswarlu S, Trimurtulu G, Jaeger W, Szekeres T (2009) Digalloylresveratrol, a new phenolic acid derivative induces apoptosis and cell cycle arrest in human HT-29 colon cancer cells. Cancer Lett 274(2): 299–304 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous