Future therapeutic directions in reverse cholesterol transport - PubMed (original) (raw)
Review
Future therapeutic directions in reverse cholesterol transport
Amit V Khera et al. Curr Atheroscler Rep. 2010 Jan.
Abstract
Despite a robust inverse association between high-density lipoprotein (HDL) cholesterol levels and atherosclerotic cardiovascular disease, the development of new therapies based on pharmacologic enhancement of HDL metabolism has proven challenging. Emerging evidence suggests that static measurement of HDL levels has inherent limitations as a surrogate for overall HDL functionality, particularly with regard to the rate of flux through the macrophage reverse cholesterol transport (RCT) pathway. Recent research has provided important insight into the molecular underpinnings of RCT, the process by which excess cellular cholesterol is effluxed from peripheral tissues and returned to the liver for ultimate intestinal excretion. This review discusses the critical importance and current strategies for quantifying RCT flux. It also highlights therapeutic strategies for augmenting macrophage RCT via three conceptual approaches: 1) improved efflux of cellular cholesterol via targeting the macrophage; 2) enhanced cholesterol efflux acceptor functionality of circulating HDL; and 3) increased hepatic uptake and biliary/intestinal excretion.
Conflict of interest statement
Disclosure Dr. Rader serves as a consultant to several companies that market or are developing therapies targeting HDL or RCT, including Abbott, AstraZeneca, Bristol-Myers-Squibb, Eli Lilly, Johnson & Johnson, Merck & Co, Novartis, and Resverlogix. No other potential conflicts of interest relevant to this article were reported.
Figures
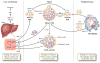
Fig. 1
Physiology of macrophage reverse cholesterol transport. Both the liver and intestine synthesize apolipoprotein A-I (ApoA-I), which is secreted in lipid-poor form. These particles are lipidated with both phospholipids and free cholesterol via the hepatocyte ATP-binding cassette A1 (ABCA1) transporter to form nascent high-density lipoprotein (HDL). In peripheral tissues, these HDL particles obtain additional free cholesterol via the macrophage ABCA1 transporter. Lecithin cholesterol acyltransferase (LCAT) esterifies free cholesterol to cholesteryl esters, generating mature HDL. These larger HDL particles serve as additional acceptors of cholesterol efflux via the macrophage ATP-binding cassette G1 (ABCG1) pathway. Liver X receptor (LXR) regulates the expression of both ABCA1 and ABCG1 in the macrophage. HDL can be remodeled by lipases such as hepatic lipase and endothelial lipase (EL), which hydrolyze HDL triglyceride and phospholipid, respectively. Mature HDL can transport its cholesterol directly to the liver via the hepatic scavenger receptor class B type 1 (SR-B1) receptor. Alternatively, cholesteryl ester transfer protein (CETP) can mediate transfer of cholesteryl esters from HDL particles to apoB-containing lipoproteins with subsequent uptake in the liver via the low-density lipoprotein receptor (LDLR). This “indirect” pathway is thought to predominate in humans. Hepatic cholesterol is secreted into the bile via the ABCG5 and ABCG8 transporters. Some reenters the circulation via intestinal reabsorption, and the remainder is excreted into the feces. Recent evidence suggests that the intestine may be able to directly excrete cholesterol, bypassing the liver entirely. VLDL—very low density lipoprotein
Similar articles
- Cholesterol efflux and reverse cholesterol transport.
Favari E, Chroni A, Tietge UJ, Zanotti I, Escolà-Gil JC, Bernini F. Favari E, et al. Handb Exp Pharmacol. 2015;224:181-206. doi: 10.1007/978-3-319-09665-0_4. Handb Exp Pharmacol. 2015. PMID: 25522988 Review. - Seeking novel targets for improving in vivo macrophage-specific reverse cholesterol transport: translating basic science into new therapies for the prevention and treatment of atherosclerosis.
Julve J, Llaverias G, Blanco-Vaca F, Escolà-Gil JC. Julve J, et al. Curr Vasc Pharmacol. 2011 Mar;9(2):220-37. doi: 10.2174/157016111794519264. Curr Vasc Pharmacol. 2011. PMID: 21143175 Review. - HDL Cholesterol Efflux Capacity: Cardiovascular Risk Factor and Potential Therapeutic Target.
Bhatt A, Rohatgi A. Bhatt A, et al. Curr Atheroscler Rep. 2016 Jan;18(1):2. doi: 10.1007/s11883-015-0554-1. Curr Atheroscler Rep. 2016. PMID: 26710794 Review. - The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis.
Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. Rader DJ, et al. J Lipid Res. 2009 Apr;50 Suppl(Suppl):S189-94. doi: 10.1194/jlr.R800088-JLR200. Epub 2008 Dec 8. J Lipid Res. 2009. PMID: 19064999 Free PMC article.
Cited by
- A big role for small RNAs in HDL homeostasis.
Ouimet M, Moore KJ. Ouimet M, et al. J Lipid Res. 2013 May;54(5):1161-7. doi: 10.1194/jlr.R036327. Epub 2013 Mar 18. J Lipid Res. 2013. PMID: 23509405 Free PMC article. Review. - HDL Cholesterol Story Is Dead: Long Live HDL!
Simha V, Kudva YC. Simha V, et al. Diabetes. 2016 Oct;65(10):2826-8. doi: 10.2337/dbi16-0039. Diabetes. 2016. PMID: 27659225 Free PMC article. No abstract available. - Differential regulation of gene expression by LXRs in response to macrophage cholesterol loading.
Ignatova ID, Angdisen J, Moran E, Schulman IG. Ignatova ID, et al. Mol Endocrinol. 2013 Jul;27(7):1036-47. doi: 10.1210/me.2013-1051. Epub 2013 May 17. Mol Endocrinol. 2013. PMID: 23686114 Free PMC article. - Intravenous Curcumin Mitigates Atherosclerosis Progression in Cholesterol-Fed Rabbits.
Momtazi-Borojeni AA, Zabihi NA, Bagheri RK, Majeed M, Jamialahmadi T, Sahebkar A. Momtazi-Borojeni AA, et al. Adv Exp Med Biol. 2021;1308:45-54. doi: 10.1007/978-3-030-64872-5_5. Adv Exp Med Biol. 2021. PMID: 33861436 - HDL, Atherosclerosis, and Emerging Therapies.
Hafiane A, Genest J. Hafiane A, et al. Cholesterol. 2013;2013:891403. doi: 10.1155/2013/891403. Epub 2013 May 28. Cholesterol. 2013. PMID: 23781332 Free PMC article.
References
- Miller GJ, Miller NE. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975;1:16–19. - PubMed
- Gordon DJ. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. - PubMed
- Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA. 2007;298:786–798. - PubMed
- Briel M, Ferreira-Gonzalez I, You JJ, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 2009;338:b92. This provocative meta-analysis suggested that increased HDL-C levels explain minimal variability with regard to clinical outcomes, reinforcing the need to move beyond simple measures of HDL-C quantity as surrogates of clinical efficacy. - PMC - PubMed
- Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the atheroprotective effect of high density lipoproteins. J Intern Med. 2008;263:256–273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical