Abeta-mediated NMDA receptor endocytosis in Alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61 - PubMed (original) (raw)
Abeta-mediated NMDA receptor endocytosis in Alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61
Pradeep Kurup et al. J Neurosci. 2010.
Abstract
Amyloid beta (Abeta) is involved in the etiology of Alzheimer's disease (AD) and may contribute to cognitive deficits by increasing internalization of ionotropic glutamate receptors. Striatal-enriched protein tyrosine phosphatase 61 (STEP(61)), which is targeted in part to the postsynaptic terminal, has been implicated in this process. Here we show that STEP(61) levels are progressively increased in the cortex of Tg2576 mice over the first year, as well as in prefrontal cortex of human AD brains. The increased STEP(61) was associated with greater STEP activity, dephosphorylation of phospho-tyr(1472) of the NR2B subunit, and decreased NR1 and NR2B subunits on neuronal membranes. Treatment with Abeta-enriched medium also increased STEP(61) levels and decreased NR1/NR2B abundance in mouse cortical cultures as determined by biotinylation experiments. In STEP knock-out cultures, Abeta treatment failed to induce NMDA receptor internalization. The mechanism for the increase in STEP(61) levels appears to involve the ubiquitin proteasome system. Blocking the proteasome resulted in elevated levels of STEP(61). Moreover, STEP(61)-ubiquitin conjugates were increased in wild-type cortical slices upon Abeta treatment as well as in 12 month Tg2576 cortex. These findings reveal a novel mechanism by which Abeta-mediated accumulation of STEP(61) results in increased internalization of NR1/NR2B receptor that may contribute to the cognitive deficits in AD.
Figures
Figure 1.
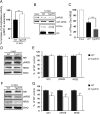
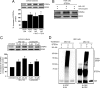
STEP61 levels are increased in Tg2576 mice and human AD brains. A, Immunoblot of STEP61 and total Aβ levels in WT and Tg2576 mice cortex at 3, 6, 9, and 12 months (MO) of age. B, Quantitation of STEP61 protein levels in WT and Tg2576 mice normalized to ERK2 levels. STEP61 levels were increased in 6-, 9-, and 12-month-old Tg2576 mice compared to age matched WT controls (*p < 0.05; two-way ANOVA with post hoc Tukey's test; n = 4;). C, STEP61 is increased in human AD brain samples. Representative immunoblot of prefrontal cortex from control and AD brains probed with anti-human STEP antibody. D, Quantitation of STEP61 control (n = 10) and AD (n = 18) groups normalized to β-actin levels. Data are expressed as percentage increase in STEP61 compared to controls.
Figure 2.
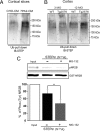
Increased STEP61 activity is associated with decreased synaptosomal membrane-associated NR1/NR2B receptor subunits in 12-month-old Tg2576 mice. A, Immunoprecipitated STEP61 from cortical brain lysates of 12-month-old (12 MO) Tg2576 mice has more phosphatase activity against pNPP than STEP61 from age-matched WT mice (**p < 0.01; Student's t test; n = 3). B, C, Representative immunoblot (B) and quantitative analysis (C) show greater dephosphorylation of Fyn-phosphorylated GST-NR2B with immunoprecipitated STEP61 from Tg2576 mice relative to WT controls. Phospho-(pY14721472)-NR2B levels were detected using a specific antibody against the pY1472 site of NR2B. Quantified pY1472 NR2B (pNR2B) levels were normalized relative to Fyn-phosphorylated GST-NR2B levels (input; **p < 0.01; Student's t test; n = 3). D, F, Representative immunoblots showing NR1, pY1472 NR2B, and NR2B levels in LP1 fractions of 3 month (D) and 12 month (F) Tg2576 mice. E, G, Quantitative analysis revealed no significant decrease in NR1, pY1472 NR2B and NR2B in 3-month-old Tg2576 mice (E), but significant decreases at 12 months compared to age-matched WT mice (G; **p < 0.01; Student's t test; n = 6).
Figure 3.
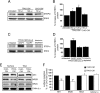
Aβ-enriched, but not control, medium increases STEP61 abundance in cortical neurons. A, Primary cortical cultures were treated with normal medium (untreated), with different amounts of 7PA2-CM (Aβ-enriched), or with CHO-CM (no Aβ) for 2 h. Representative immunoblot shows increased STEP61 levels with 7PA2-CM treatment but not with CHO-CM treatment. B, Quantitative analysis reveals a dose-dependent increase of STEP61 levels after treatment with 7PA2-CM but not with CHO-CM after normalization to total ERK2 levels (*p < 0.05; 7PA2-CM vs untreated; one-way ANOVA with post hoc Tukey's test; n = 5). C, Cortical cultures were treated with normal medium (untreated), with 7PA2-CM with or without the γ-secretase inhibitor DAPT (250 n
m
), or with 7PA2-CM immunodepleted of Aβ using 6E10 antibody. A representative immunoblot is shown. D, Quantitative analysis shows that DAPT treatment and immunodepleted medium blocks the increase in STEP61. Normalization was performed using total ERK2 (**p < 0.01; one-way ANOVA with post hoc Tukey's test; n = 3). E, Surface biotinylation of cortical cultures is shown after treatment with CHO-CM or 7PA2-CM. Western blots performed on membrane-associated fractions. F, Surface and total NR1, NR2A, NR2B, and GABAAβ 2/3 receptor levels were quantified (**p < 0.01; 7PA2-CM vs CHO-CM; Student's t test; n = 3).
Figure 4.
Aβ-enriched medium increases STEP61 levels and STEP61 dephosphorylation, and decreases NR2B abundance in membrane fractions of cortical slices. Cortical slices were incubated with 7PA2-CM (Aβ-enriched) at different time points and processed to obtain membrane fractions (P2). A–C, Samples were analyzed by immunoblotting with anti-STEP antibody (A), anti-pSer221 STEP antibody (B), or anti-NR2B antibody (C). Representative immunoblots are shown along with quantitation of cumulative data. Normalization was performed using total ERK2 (*p < 0.05; one-way ANOVA with post hoc Tukey's test; n = 3).
Figure 5.
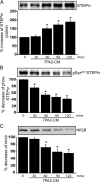
Aβ treatment in STEP KO cultures does not alter surface NR1/NR2B levels. Cortical cultures from WT and STEP KO mice were treated with 7PA2-CM (Aβ-enriched), and biotinylated surface proteins were analyzed by immunoblotting. A, Representative Western blots of NR1 and quantitation are shown. The surface NR1 receptors are normalized to total NR1 levels, and WT CHO (lane 1) served as control for all other treatments (*p < 0.05; **_p_ < 0.01; one-way ANOVA with _post hoc_ Tukey's test; _n_ = 5). STEP KO cultures have higher baseline levels of surface NR1 receptors (KO CHO vs WT CHO; **_p_ < 0.01; _n_ = 5). Aβ treatment decreased surface NR1 in WT cultures (**_p_ < 0.01; Aβ vs CHO in WT; _n_ = 5), but not in STEP KO cultures (_p_ > 0.05; Aβ vs CHO in STEP KO; n = 5). B, Representative Western blots of NR2B and quantitation are shown. The surface NR2B receptors are normalized to total NR2B levels, and WT CHO (lane 1) served as control for all other treatments (*p < 0.05; **_p_ < 0.01; one-way ANOVA with _post hoc_ Tukey's test; _n_ = 5). STEP KO cultures have higher baseline levels of surface NR2B receptors (KO CHO vs WT CHO; **_p_ < 0.01; _n_ = 5). Aβ treatment decreased surface NR2B in WT cultures (**_p_ < 0.01; Aβ vs CHO in WT; _n_ = 5), but not in STEP KO cultures (_p_ > 0.05; Aβ vs CHO in STEP KO; n = 5).
Figure 6.
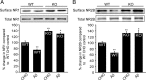
Increased STEP61 levels do not require transcription or translation but require inhibition of the ubiquitin proteasome system. A, Cortical cultures were pretreated with actinomycin D (20 μ
m
) or cycloheximide (100 μ
m
) for 20 min and then incubated with 7PA2-CM (Aβ-enriched) for 2 h. Normalization was performed using total ERK2 (*p < 0.05, CHO-CM vs other groups; one-way ANOVA with post hoc Tukey's test; n = 3). B, HEK-293T cells were transfected with STEP61 cDNA and incubated for 36 h. Cells were treated with vehicle (0.1% DMSO), the proteasome inhibitor MG-132 (10 μ
m
), or the lysosomal inhibitor chloroquine (500 μ
m
) for 4 h. Representative immunoblot shows an increase in STEP immunoreactivity in the presence of MG-132. C, Cortical cultures were incubated with two structurally different proteasome inhibitors: MG-132 (10 μ
m
and 20 μ
m
) and lactacystin (5 μ
m
and 10 μ
m
) for 2 h. Quantification showed a dose-dependent increase in STEP61 after exposure to both inhibitors and normalization to ERK2 (*p < 0.05, treatment vs control; one-way ANOVA with post hoc Tukey's test; n = 3). D, HEK cells were transfected with STEP61, STEP61 plus HA-tagged ubiquitin cDNAs, or HA-tagged ubiquitin alone. After 36 h, cells were treated with MG-132, immunoprecipitated with STEP antibody, and probed with anti-HA (left) or anti-STEP antibody (right). Higher molecular weight HA and STEP immunoreactivity was observed from cells that coexpressed STEP61 and HA-ubiquitin.
Figure 7.
Ubiquitin-conjugated STEP61 levels are increased in 7PA2-CM-treated cortical slices and in 12-month Tg2576 brains. A, Ubiquitin-conjugated proteins were isolated by affinity chromatography from rat cortical slices after treatment with control CHO-CM or 7PA2-CM (Aβ-enriched) for 2 h. Immunoblotting shows that ubiquitinated STEP61 levels are increased after treatment with 7PA2-CM. B, Ubiquitin-conjugated proteins were isolated from cortical lysates of WT and Tg2576 mice (3 and 12 months) and probed with an anti-STEP antibody. Immunoblot shows that increased levels of ubiquitinated STEP61 at 12 months in Tg2576 cortex. C, HEK cells were transfected with STEP61 (ser221 to ala) cDNA and treated with or without MG-132 (4 h; 10 μ
m
) and immunoprecipitated with anti-STEP antibody. The immunoprecipitated complex was assayed in vitro for phosphatase activity using Fyn-phosphorylated GST-phospho-NR2B as a substrate. Dephosphorylation of pY1472 NR2B (pNR2B) was assessed by immunoblotting with a p-tyr1472-specific antibody and normalized to the initial Fyn-phosphorylated GST-phospho-NR2B levels (input). Inhibition of the proteasome with MG-132 results in greater dephosphorylation of Fyn-phosphorylated GST-phospho-NR2B relative to control (*p < 0.05, Student's t test; n = 3).
Similar articles
- Reduced levels of the tyrosine phosphatase STEP block β amyloid-mediated GluA1/GluA2 receptor internalization.
Zhang Y, Kurup P, Xu J, Anderson GM, Greengard P, Nairn AC, Lombroso PJ. Zhang Y, et al. J Neurochem. 2011 Nov;119(3):664-72. doi: 10.1111/j.1471-4159.2011.07450.x. Epub 2011 Sep 21. J Neurochem. 2011. PMID: 21883219 Free PMC article. - The role of STEP in Alzheimer's disease.
Kurup P, Zhang Y, Venkitaramani DV, Xu J, Lombroso PJ. Kurup P, et al. Channels (Austin). 2010 Sep-Oct;4(5):347-50. doi: 10.1523/JNEUROSCI.0157-10.2010. Epub 2010 Sep 6. Channels (Austin). 2010. PMID: 20699650 Free PMC article. - Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer's disease mouse model.
Zhang Y, Kurup P, Xu J, Carty N, Fernandez SM, Nygaard HB, Pittenger C, Greengard P, Strittmatter SM, Nairn AC, Lombroso PJ. Zhang Y, et al. Proc Natl Acad Sci U S A. 2010 Nov 2;107(44):19014-9. doi: 10.1073/pnas.1013543107. Epub 2010 Oct 18. Proc Natl Acad Sci U S A. 2010. PMID: 20956308 Free PMC article. - Current Research Therapeutic Strategies for Alzheimer's Disease Treatment.
Folch J, Petrov D, Ettcheto M, Abad S, Sánchez-López E, García ML, Olloquequi J, Beas-Zarate C, Auladell C, Camins A. Folch J, et al. Neural Plast. 2016;2016:8501693. doi: 10.1155/2016/8501693. Epub 2016 Jan 3. Neural Plast. 2016. PMID: 26881137 Free PMC article. Review. - Taking STEPs forward to understand fragile X syndrome.
Goebel-Goody SM, Lombroso PJ. Goebel-Goody SM, et al. Results Probl Cell Differ. 2012;54:223-41. doi: 10.1007/978-3-642-21649-7_12. Results Probl Cell Differ. 2012. PMID: 22009355 Free PMC article. Review.
Cited by
- Role of Striatal-Enriched Tyrosine Phosphatase in Neuronal Function.
Kamceva M, Benedict J, Nairn AC, Lombroso PJ. Kamceva M, et al. Neural Plast. 2016;2016:8136925. doi: 10.1155/2016/8136925. Epub 2016 Apr 12. Neural Plast. 2016. PMID: 27190655 Free PMC article. Review. - Striatal-enriched protein tyrosine phosphatase (STEP) knockout mice have enhanced hippocampal memory.
Venkitaramani DV, Moura PJ, Picciotto MR, Lombroso PJ. Venkitaramani DV, et al. Eur J Neurosci. 2011 Jun;33(12):2288-98. doi: 10.1111/j.1460-9568.2011.07687.x. Epub 2011 Apr 19. Eur J Neurosci. 2011. PMID: 21501258 Free PMC article. - Protein tyrosine phosphatases as potential therapeutic targets.
He RJ, Yu ZH, Zhang RY, Zhang ZY. He RJ, et al. Acta Pharmacol Sin. 2014 Oct;35(10):1227-46. doi: 10.1038/aps.2014.80. Epub 2014 Sep 15. Acta Pharmacol Sin. 2014. PMID: 25220640 Free PMC article. Review. - Increasing the Receptor Tyrosine Kinase EphB2 Prevents Amyloid-β-induced Depletion of Cell Surface Glutamate Receptors by a Mechanism That Requires the PDZ-binding Motif of EphB2 and Neuronal Activity.
Miyamoto T, Kim D, Knox JA, Johnson E, Mucke L. Miyamoto T, et al. J Biol Chem. 2016 Jan 22;291(4):1719-1734. doi: 10.1074/jbc.M115.666529. Epub 2015 Nov 20. J Biol Chem. 2016. PMID: 26589795 Free PMC article. - Neuronal surface P antigen (NSPA) modulates postsynaptic NMDAR stability through ubiquitination of tyrosine phosphatase PTPMEG.
Espinoza S, Arredondo SB, Barake F, Carvajal F, Guerrero FG, Segovia-Miranda F, Valenzuela DM, Wyneken U, Rojas-Fernández A, Cerpa W, Massardo L, Varela-Nallar L, González A. Espinoza S, et al. BMC Biol. 2020 Nov 6;18(1):164. doi: 10.1186/s12915-020-00877-2. BMC Biol. 2020. PMID: 33158444 Free PMC article.
References
- Altar CA, Jurata LW, Charles V, Lemire A, Liu P, Bukhman Y, Young TA, Bullard J, Yokoe H, Webster MJ, Knable MB, Brockman JA. Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiatry. 2005;58:85–96. - PubMed
- Botto L, Masserini M, Palestini P. Changes in the composition of detergent-resistant membrane domains of cultured neurons following protein kinase C activation. J Neurosci Res. 2007;85:443–450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH052711-15/MH/NIMH NIH HHS/United States
- AG05138/AG/NIA NIH HHS/United States
- K02 MH001527-10/MH/NIMH NIH HHS/United States
- R01 MH052711-17/MH/NIMH NIH HHS/United States
- K02 MH001527/MH/NIMH NIH HHS/United States
- P01 AG009464/AG/NIA NIH HHS/United States
- AG02219/AG/NIA NIH HHS/United States
- P01 AG002219/AG/NIA NIH HHS/United States
- MH52711/MH/NIMH NIH HHS/United States
- P50 AG005138/AG/NIA NIH HHS/United States
- AG09464/AG/NIA NIH HHS/United States
- R01 MH052711/MH/NIMH NIH HHS/United States
- MH01527/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases