Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease - PubMed (original) (raw)
Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease
Kevin C Miranda et al. Kidney Int. 2010 Jul.
Abstract
Urinary exosomes or microvesicles are being studied intensively to identify potential new biomarkers for renal disease. We sought to identify whether these microvesicles contain nucleic acids. We isolated microvesicles from human urine in the same density range as that previously described for urinary exosomes and found them to have an RNA integrity profile similar to that of kidney tissue, including 18S and 28S rRNA. This profile was better preserved in urinary microvesicles compared with whole cells isolated from urine, suggesting that microvesicles may protect RNA during urine passage. We were able to detect mRNA in the human urinary microvesicles encoding proteins from all regions of the nephron and the collecting duct. Further, to provide a proof of principle, we found that microvesicles isolated from the urine of the V-ATPase B1 subunit knockout mice lacked mRNA of this subunit while containing a normal amount of the B2 subunit and aquaporin 2. The microvesicles were found to be contaminated with extraneous DNA potentially on their surface; therefore, we developed a rapid and reliable means to isolate nucleic acids from within urine microvesicles devoid of this extraneous contamination. Our study provides an experimental strategy for the routine isolation and use of urinary microvesicles as a novel and non-invasive source of nucleic acids to further renal disease biomarker discovery.
Figures
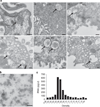
Figure 1. Electron microscopy of urinary microvesicles
(a) Multivesicular bodies (MVBs) can be identified in various regions of the nephron and collecting duct (see arrows). Bar = 200 nm for A, C, D, E, F; 500 nm for B. (b) Human urinary microvesicles isolated using differential ultracentrifugation and imaged via transmission electron microscopy using phosphotungstic acid as a stain. Bar = 200 nm. (c) Percoll gradient analysis of urinary microvesicles shows that RNA-containing microvesicles are within the density range for urinary microvesicles previously characterized as exosomes. CD-IC, collecting duct intercalated cell; CD-PC, collecting duct principal cell; Podo, podocyte; PT, proximal tubule; TAL, thick ascending limb; TDL, thin descending limb.
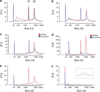
Figure 2. Analysis of nucleic acids associated with urinary microvesicles using the Agilent Bioanalyzer
(a) Plot showing that microvesicles may co-isolate with extraneous DNA that can be removed by DNase digestion of the microvesicle pellet prior to lysis and nucleic acid extraction. Red — profile without DNase digestion, blue — profile with DNase digestion. (b) Plot showing that microvesicles do not co-isolate with detectable levels of extraneous RNA. Red — without RNase digestion, blue — with RNase digestion. (c) RNA isolated from rat kidney (red) and microvesicles (blue) exhibited a very similar profile, including the presence of 18S and 28S rRNA peaks. Both samples underwent processing using the RNeasy Plus Micro kit to remove genomic DNA (gDNA) contamination. (d) Urinary microvesicles contain a prominent ‘small RNA’ peak between 25–200 nt when miRNA isolation techniques are used. Red — kidney RNA isolated using RNeasy Plus Micro kit using the miRNA extraction method, blue — microvesicle RNA isolated with RNeasy Plus Micro kit using the miRNA extraction method. (e) Nucleic acids were isolated from microvesicles that had undergone RNase and DNase digestion on the outside before microvesicle lysis. During RNA extraction using the RNeasy Micro kit, half of the samples underwent on-column RNase digestion (see Materials and methods) while the other half underwent the same on-column incubation without the presence of RNase. Results revealed that RNase digestion was able to remove the majority of the profile, suggesting that RNA is the major nucleic acid within urinary microvesicles. Red — nucleic acid profile without intra-microvesicular RNase digestion, blue — nucleic acid profile with intra-microvesicular RNase digestion. (f) Further digestion with DNase following RNase digestion revealed that the remaining peak could be further reduced, suggesting that some material prone to DNase digestion remained in the sample potentially representing intra-exosomal DNA. Red — nucleic acid profile following intra-microvesicular on-column RNase digestion alone, blue — nucleic acid profile following both intra-microvesicular on-column RNase and DNase digestion. 18S and 28S rRNA peaks are indicated in (a). The peak at 25 nt represents an internal standard.
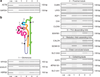
Figure 3. Urinary microvesicles contain messenger RNA (mRNA) transcripts encoding specific genes from various regions of the nephron and the collecting duct
(a) Bioanalyzer-generated ‘Pseudo gel’ profiles of the positive identification of RiboAmp-amplified mRNA transcripts for β-actin (ACTB) and glyceraldehyde 3-phosphate (GAPDH) in urinary microvesicles from four subjects by RT-PCR. (b) Cartoon of the nephron and collecting duct highlighting its functionally distinct regions. (c) The positive identification of (1) Glomerulus: NPHS2 — podocin, LGALS1 — Galectin-1, HSPG2 — heparan sulfate proteoglycan; (2) proximal tubule: CUBN — cubilin, LRP2 — megalin, AQP1 — aquaporin 1, CA4 — carbonic anhydrase 4, CLCN5 — chloride channel protein 5, (3) thin descending limb: BDKRB1 — bradykinin B1 receptor; (4) medullary thick ascending limb: CALCR — calcitonin receptor, SCNN1D — amiloride-sensitive sodium channel subunit delta; (5) distal convoluted tubule: SLC12A3 — thiazide-sensitive sodium-chloride cotransporter; (6) collecting ducts: AQP2 — aquaporin 2, ATP6V1B1 — V-ATPase B1 subunit, SLC12A1 — kidney-specific Na–K–Cl symporter via RT-PCR of RiboAmped mRNA from urinary exosomes.
Figure 4. Analysis of gene expression by RT-PCR and real-time PCR in the V-ATPase B1 knockout model of renal acidosis
(a) Analysis of the V-ATPase B1 subunit and AQP2 mRNA by RT-PCR in V-ATPase B1 KO (B1 −/−) and wild-type (B1 +/+) mice reveals that similar results can be obtained via both kidney and microvesicle RNA analysis. (b) Using urinary microvesicles, real-time PCR analysis demonstrated that the expression of the V-ATPase B2 subunit could be analyzed non-invasively to demonstrate that the B2 subunit expression is not affected by the absence of the V-ATPase B1. These results were consistent with those obtained via the corresponding kidney-derived RNA analysis. NS, not statistically significant.
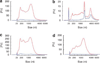
Figure 5. RNA extracted from whole urine cells and debris has a different RNA profile from that of tissue and urinary microvesicles
(a) Analysis of RNA isolated from whole urine (exclusive of microvesicles that are not captured by the isolation technique) showed that a large yield of nucleic acids can be isolated (see the red profile). Processing of the isolated nucleic acids using the RNeasy Plus Micro kit (which removes gDNA) reveals that the majority of nucleic acids isolated using the ZR urine RNA isolation kit is DNA and the remaining RNA lacks rRNA peaks found in tissue and urinary exosomes. Red — nucleic acids isolated from whole urine without gDNA removal, blue — nucleic acids isolated from whole urine post gDNA removal using the RNeasy Plus Micro kit. (b) Isolation of microvesicles from the same urine sample revealed that the microvesicles retained a normal total RNA profile suggesting that RNA within whole cells may be less stable than that contained in urinary microvesicles. Red — without removal of gDNA, blue — sample processed using the RNeasy Plus Micro kit to remove contaminating gDNA. (c) Isolation of nucleic acids from the pellet formed during the 300 g spin revealed that the nucleic acid profile was different from that of microvesicles and that it contained a large amount of gDNA following processing using the RNeasy Plus Micro kit. Red — nucleic acids isolated from the 300 g pellet without gDNA removal, blue — nucleic acid isolated from the 300 g pellet post gDNA removal using the RNeasy Plus Micro kit. (d) Isolation of nucleic acids from pellets formed during the 17,000 g spin revealed that the nucleic acid profile was different to microvesicles and that it contained a large amount of gDNA following processing using the RNeasy Plus Micro kit. Red — nucleic acids isolated from the 17,000 g pellet without gDNA removal, blue — nucleic acids isolated from the 17,000 g pellet post gDNA removal using the RNeasy Plus Micro kit.
Figure 6. Isolation of microvesicles using filtration concentrators reveals that this may be a rapid technique to isolate intact microvesicles for nucleic acid extraction
(a) A sample of 75 ml human urine was subjected to the initial processing steps of 300 g, 17,000 g centrifugation followed by 0.8 µm filtration and was then processed using ultracentrifugation (blue) or using 100 kDa MWCO filters (red). The results revealed that a similar profile was obtained using both extraction methods with minimal degradation of the RNA revealing that filtration concentrators may be a fast and reliable way to isolate urinary microvesicles. Comparison of the ‘normal’ 300 g, 17,000 g spin and 0.8 µm filtration steps (red) with just a 0.8 µm filtration step (blue) followed by (b) ultracentrifugation or (c) filtration concentrators revealed that the 300 g and 17,000 g spins were not crucial for the removal of cell debris and whole cells as no change in profile was observed, further simplifying the isolation technique and the time taken to isolate exosomes. All samples underwent extra-exosomal RNase and DNase digestion before microvesicular lysis.
Similar articles
- Urinary extracellular microvesicles: isolation methods and prospects for urinary proteome.
Wang D, Sun W. Wang D, et al. Proteomics. 2014 Aug;14(16):1922-32. doi: 10.1002/pmic.201300371. Epub 2014 Jul 28. Proteomics. 2014. PMID: 24962155 Review. - Comparison of three methods for isolation of urinary microvesicles to identify biomarkers of nephrotic syndrome.
Rood IM, Deegens JK, Merchant ML, Tamboer WP, Wilkey DW, Wetzels JF, Klein JB. Rood IM, et al. Kidney Int. 2010 Oct;78(8):810-6. doi: 10.1038/ki.2010.262. Epub 2010 Aug 4. Kidney Int. 2010. PMID: 20686450 - Discrimination of urinary exosomes from microvesicles by lipidomics using thin layer liquid chromatography (TLC) coupled with MALDI-TOF mass spectrometry.
Singhto N, Vinaiphat A, Thongboonkerd V. Singhto N, et al. Sci Rep. 2019 Sep 25;9(1):13834. doi: 10.1038/s41598-019-50195-z. Sci Rep. 2019. PMID: 31554842 Free PMC article. Retracted. - Prospects for urinary proteomics: exosomes as a source of urinary biomarkers.
Hoorn EJ, Pisitkun T, Zietse R, Gross P, Frokiaer J, Wang NS, Gonzales PA, Star RA, Knepper MA. Hoorn EJ, et al. Nephrology (Carlton). 2005 Jun;10(3):283-90. doi: 10.1111/j.1440-1797.2005.00387.x. Nephrology (Carlton). 2005. PMID: 15958043 Review. - Exosomes in urine biomarker discovery.
Huebner AR, Somparn P, Benjachat T, Leelahavanichkul A, Avihingsanon Y, Fenton RA, Pisitkun T. Huebner AR, et al. Adv Exp Med Biol. 2015;845:43-58. doi: 10.1007/978-94-017-9523-4_5. Adv Exp Med Biol. 2015. PMID: 25355568
Cited by
- Comparison of chromatin accessibility remodeling of granulosa cells in patients with endometrioma or pelvic/tubal infertility.
Ou S, Jiao X, Li Y, Pan P, Li R, Huang J, Sun X, Wang W, Zhang Q, Cao C, Wei L. Ou S, et al. J Assist Reprod Genet. 2024 Nov 1. doi: 10.1007/s10815-024-03302-7. Online ahead of print. J Assist Reprod Genet. 2024. PMID: 39485574 - Photosensitive Nanoprobes for Rapid Isolation and Size-Specific Enrichment of Synthetic and Extracellular Vesicle Subpopulations.
Weerakkody JS, Tseng T, Topper M, Thoduvayil S, Radhakrishnan A, Pincet F, Kyriakides TR, Gunasekara RW, Ramakrishnan S. Weerakkody JS, et al. Adv Funct Mater. 2024 Aug 22;34(34):2400390. doi: 10.1002/adfm.202400390. Epub 2024 Mar 29. Adv Funct Mater. 2024. PMID: 39372670 - Urinary Extracellular Vesicles for Non-Invasive Quantification of Principal Cell Damage in Kidney Transplant Recipients.
Svenningsen P, Maslauskiene R, Palarasah Y, Bumblyte IA, Tepel M. Svenningsen P, et al. Biomolecules. 2024 Sep 5;14(9):1124. doi: 10.3390/biom14091124. Biomolecules. 2024. PMID: 39334890 Free PMC article. - Biological functions and affected signaling pathways by Long Non-Coding RNAs in the immune system.
Ghahramani Almanghadim H, Karimi B, Valizadeh S, Ghaedi K. Ghahramani Almanghadim H, et al. Noncoding RNA Res. 2024 Sep 6;10:70-90. doi: 10.1016/j.ncrna.2024.09.001. eCollection 2025 Feb. Noncoding RNA Res. 2024. PMID: 39315339 Free PMC article. Review. - Future embracing: exosomes driving a revolutionary approach to the diagnosis and treatment of idiopathic membranous nephropathy.
Wang L, Wang J, Xu A, Wei L, Pei M, Shen T, Xian X, Yang K, Fei L, Pan Y, Yang H, Wang X. Wang L, et al. J Nanobiotechnology. 2024 Aug 8;22(1):472. doi: 10.1186/s12951-024-02633-y. J Nanobiotechnology. 2024. PMID: 39118155 Free PMC article. Review.
References
- Stoorvogel W, Kleijmeer MJ, Geuze HJ, et al. The biogenesis and functions of exosomes. Traffic. 2002;3:321–330. - PubMed
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artifacts no more. Trends Cell Biol. 2009;19:43–51. - PubMed
- Marzesco AM, Janich P, Wilsch-Bräuninger M, et al. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci. 2005;118:2849–2858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01 DK073266/DK/NIDDK NIH HHS/United States
- DK38452/DK/NIDDK NIH HHS/United States
- R56 DK042956/DK/NIDDK NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
- DK73266/DK/NIDDK NIH HHS/United States
- DK43341/DK/NIDDK NIH HHS/United States
- DK42956/DK/NIDDK NIH HHS/United States
- R37 DK042956/DK/NIDDK NIH HHS/United States
- R01 DK042956/DK/NIDDK NIH HHS/United States
- P01 DK038452/DK/NIDDK NIH HHS/United States
- DK57521/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases