Following gaze: gaze-following behavior as a window into social cognition - PubMed (original) (raw)
Following gaze: gaze-following behavior as a window into social cognition
Stephen V Shepherd. Front Integr Neurosci. 2010.
Abstract
In general, individuals look where they attend and next intend to act. Many animals, including our own species, use observed gaze as a deictic ("pointing") cue to guide behavior. Among humans, these responses are reflexive and pervasive: they arise within a fraction of a second, act independently of task relevance, and appear to undergird our initial development of language and theory of mind. Human and nonhuman animals appear to share basic gaze-following behaviors, suggesting the foundations of human social cognition may also be present in nonhuman brains.
Keywords: attention; joint attention; orienting; shared attention; social attention; theory of mind.
Figures
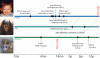
Figure 1
Ontogeny of primate gaze following. Humans, apes, and monkeys are sensitive to direct gaze at, or soon after, birth. However, their understanding of deictic gaze develops during childhood. Human gaze following arises early in life, with responses to turned heads and averted eyes arising between 2–6 months; gaze following at 10–12 months predicts language acquisition over the next year. Near the 1-year mark, human gaze following becomes more sophisticated: it is contingent on cue's eyes being open at 11 months, and on the cue having recently looked at interesting things by 14 months; by 18 months, humans follow gaze geometrically to regions beyond their immediate line of sight. By contrast, much less is known about the development of nonhuman gaze following. Apes and monkeys both appear more sensitive to head direction than to eyes. Both habituate to misleading gaze cues during adolescence, and as adults, follow gaze geometrically and from eye cues; the precise onset of these abilities is uncertain.
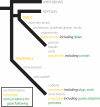
Figure 2
Phylogeny of vertebrate gaze following. Though the most robust evidence for gaze following has emerged from primates, other mammals and birds have also been found to follow gaze. Evidence comes primarily from three groups: domestic mammals including goats and dogs, captive cetaceans including dolphins and seals, and birds including corvids and ibises. Better understanding of the evolution of gaze behavior will require more comparative studies, with a particular eye toward distinguishing both the sophistication and species-specificity of gaze cue responses.
Figure 3
Lemurs use deictic social cues to guide naturalistic orienting. Upper: New technologies permit human and nonhuman gaze to be recorded during naturalistic interaction. These techniques can reveal subtle patterns of signaling which are difficult to detect in the field or evoke in the laboratory. Michael Platt and I recorded infrared video (A) of a lemur's eye, as reflected in a dichroic mirror (B), while simultaneously recording (C) the scene in front of the lemur and transmitting (D) both data sets to a computer for extraction of gaze location. Though lemurs reportedly ignore human gaze cues, they nonetheless co-oriented with one another in natural settings, and tended to follow the gaze of individuals they had recently looked at. Lower: Co-orienting statistics: across the analyzed videos, the lemur subject tended to look in the direct of the outward red lines, and avoid the direction of the inward blue lines, relative to observed lemurs’ body (larger circle) and head (smaller circle) axes (methods, Shepherd and Platt, ; results, Shepherd and Platt, 2008).
Figure 4
Humans follow gaze reflexively. (A) Faces gazing left or right were presented for 100, 300, or 700ms, followed by a response target which appeared opposite gaze four times as often as it appeared in the gazed direction. (B) For the first half-second after cue onset, subjects responded faster to targets appearing in the direction of observed gaze – despite their knowledge these targets were less likely (adapted from Driver et al., 1999).
Figure 5
Anthropoids primates follow gaze with similar sub-second dynamics. (A) Monkeys and humans performed an identical task, in which they fixated a central face gazing left or right, and then looked toward a peripheral target. The target was not predicted by the gaze direction of the cue. (B) Monkeys and humans were faster to look toward targets appearing in the direction of cue gaze, independent of whether head or eye-only cue images were used. (C) The fixation positions of monkeys and humans shifted slightly in the direction of gaze, and did so with similar time course. Such fixation shifts are thought to result from microsaccadic drift biased in the direction of attention (adapted from Deaner and Platt, 2003).
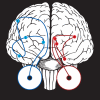
Figure 6
Potential mechanisms for gaze-following behavior. Two general pathways (shown here, schematically) could relate observed gaze to visual attention. At left, in blue, visual information travels from the retina to the lateral geniculate (1), the early visual areas (2), the social processing areas along the superior temporal sulcus (3), and finally toward attention control circuitry including the lateral intraparietal area, frontal eye fields, and superior colliculus (4). At right, in red, a hypothesized subcortical pathway travels directly from the retina to the superior colliculus (1), to the pulvinar nucleus of the thalamus (2), and to the amygdala (3). The subcortical pathway could influence attention locally in the superior colliculus or pulvinar, or via projections from amygdala to the early visual areas (4).
Similar articles
- Pre-exposure to Ambiguous Faces Modulates Top-Down Control of Attentional Orienting to Counterpredictive Gaze Cues.
Abubshait A, Momen A, Wiese E. Abubshait A, et al. Front Psychol. 2020 Sep 9;11:2234. doi: 10.3389/fpsyg.2020.02234. eCollection 2020. Front Psychol. 2020. PMID: 33013584 Free PMC article. - Spontaneous social orienting and gaze following in ringtailed lemurs (Lemur catta).
Shepherd SV, Platt ML. Shepherd SV, et al. Anim Cogn. 2008 Jan;11(1):13-20. doi: 10.1007/s10071-007-0083-6. Epub 2007 May 10. Anim Cogn. 2008. PMID: 17492318 - Neurophysiological correlates of visuospatial attention and the social dynamics of gaze processing.
Wei G, Rushby JA, De Blasio FM. Wei G, et al. Cogn Affect Behav Neurosci. 2019 Oct;19(5):1218-1230. doi: 10.3758/s13415-019-00728-w. Cogn Affect Behav Neurosci. 2019. PMID: 31187442 - Gaze following: A socio-cognitive skill rooted in deep time.
Zeiträg C, Jensen TR, Osvath M. Zeiträg C, et al. Front Psychol. 2022 Dec 2;13:950935. doi: 10.3389/fpsyg.2022.950935. eCollection 2022. Front Psychol. 2022. PMID: 36533020 Free PMC article. Review. - The developmental origins of naïve psychology in infancy.
Poulin-Dubois D, Brooker I, Chow V. Poulin-Dubois D, et al. Adv Child Dev Behav. 2009;37:55-104. doi: 10.1016/s0065-2407(09)03702-1. Adv Child Dev Behav. 2009. PMID: 19673160 Review.
Cited by
- Differences in gaze anticipation for locomotion with and without vision.
Authié CN, Hilt PM, N'Guyen S, Berthoz A, Bennequin D. Authié CN, et al. Front Hum Neurosci. 2015 Jun 8;9:312. doi: 10.3389/fnhum.2015.00312. eCollection 2015. Front Hum Neurosci. 2015. PMID: 26106313 Free PMC article. - Neuroethology of primate social behavior.
Chang SW, Brent LJ, Adams GK, Klein JT, Pearson JM, Watson KK, Platt ML. Chang SW, et al. Proc Natl Acad Sci U S A. 2013 Jun 18;110 Suppl 2(Suppl 2):10387-94. doi: 10.1073/pnas.1301213110. Epub 2013 Jun 10. Proc Natl Acad Sci U S A. 2013. PMID: 23754410 Free PMC article. - Social interaction networks in the primate brain.
Freiwald WA. Freiwald WA. Curr Opin Neurobiol. 2020 Dec;65:49-58. doi: 10.1016/j.conb.2020.08.012. Epub 2020 Oct 13. Curr Opin Neurobiol. 2020. PMID: 33065333 Free PMC article. Review. - Neurocomputational Nosology: Malfunctions of Models and Mechanisms.
Barack DL, Platt ML. Barack DL, et al. Front Psychol. 2016 May 3;7:602. doi: 10.3389/fpsyg.2016.00602. eCollection 2016. Front Psychol. 2016. PMID: 27199835 Free PMC article. Review. - Racial group membership is associated to gaze-mediated orienting in Italy.
Pavan G, Dalmaso M, Galfano G, Castelli L. Pavan G, et al. PLoS One. 2011;6(10):e25608. doi: 10.1371/journal.pone.0025608. Epub 2011 Oct 4. PLoS One. 2011. PMID: 21991323 Free PMC article.
References
LinkOut - more resources
Full Text Sources