Genetics, pathogenesis and clinical interventions in type 1 diabetes - PubMed (original) (raw)
Review
Genetics, pathogenesis and clinical interventions in type 1 diabetes
Jeffrey A Bluestone et al. Nature. 2010.
Abstract
Type 1 diabetes is an autoimmune disorder afflicting millions of people worldwide. Once diagnosed, patients require lifelong insulin treatment and can experience numerous disease-associated complications. The last decade has seen tremendous advances in elucidating the causes and treatment of the disease based on extensive research both in rodent models of spontaneous diabetes and in humans. Integrating these advances has led to the recognition that the balance between regulatory and effector T cells determines disease risk, timing of disease activation, and disease tempo. Here we describe current progress, the challenges ahead and the new interventions that are being tested to address the unmet need for preventative or curative therapies.
Figures
Figure 1. Markers of diabetes
a, Typical child followed from birth until development of diabetes in the DAISY (Diabetes Auto Immunity Study in the Young;
http://www.uchsc.edu/misc/diabetes/Teddy/DAISY/DAISY\_home.htm
) study (M. Rewers, unpublished work), expressing multiple autoantibodies (GAD, the islet cell antibody ICA512 and low-level insulin autoantibodies). b, Islet invasion by lymphocytes of NOD mice is asynchronous during progression to diabetes, often with a mixture of normal islets, peri-insulitis, intra-islet insulitis, and complete β-cell destruction. c, Pathology of the pancreas in a long-term type 1 diabetic (the nPOD program, pancreas 6028, see
). The section shows lobular areas in which all β-cells (insulin-producing) in all islets have been destroyed (pseudoatrophic islets in which only glucagon, somatostatin, and pancreatic polypeptide cells are present) juxtaposed with regions in which all islets contain insulin-containing β-cells.
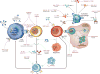
Figure 2. Immunologic history of type 1 diabetes
An as-yet-undefined immunologic insult occurs in an individual with genetic predisposition and initiates a chronic low-grade immunologic process (priming). The initiating events involve infiltration of innate immune cells (such as monocytes and natural killer cells with autoreactive B cells) (orange ovals) into the pancreatic islets. The principal site of antigen presentation is thought to be the pancreatic lymph node where islet antigens are presented by antigen-presenting cells (white ovals) to T cells (brown dots). Blue ovals are antigen-presenting cells loaded with islet antigens. B cells (green dots) and dendritic cells may be among the early antigen-presenting cells. The cellular infiltration of islets ensues but the insulitis is uneven. Islets with infiltration may be situated near to islets without cells. The process specifically targets insulin-producing β-cells (light blue circles), while other endocrine cells (red circles) within the islet are spared. In the lymph nodes, the cycle of antigen presentation, activation of adaptive immune cells, licensing of effector T cells and epitope spreading continues with the loss of β-cells over time. There is evidence for a regenerative attempt of β-cells in the midst of the islet inflammation (dark blue circles). Tertiary lymphoid organs are thought to develop within the islets, which may lead to amplification of the adaptive immune response. Regulatory T cells (yellow dots) may arrest this process in its early and late stages but are not able to contain the amplified process in the late stages despite an increase in their numbers. With continued loss of β-cells, hyperglycaemia can be detected. The loss of metabolic function at presentation may be both functional and anatomic, because immune therapies can restore cells that have lost the capacity to produce insulin but have not been destroyed. Without intervention, however, β-cell loss continues.
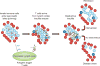
Figure 3. Immune system balance is key to disease pathogenesis
This schematic illustrates the fine balance of immune regulation versus pathogenesis, highlighting a number of genes that are likely to influence the balance through effects on central and peripheral tolerance and the environmental factors that control immunity. The key cell types that affect the balance locally during immune responses are listed (with the regulatory cytokines and proteins given in parentheses). iNOS, inducible nitric oxide synthase. IDO, indoleamine-2,3 dioxygenase.
Figure 4. Targets of immune intervention in type 1 diabetes
This schematic provides an overview of the pathogenesis of type 1 diabetes, highlighting a number of key pathways that are being targeted by current therapeutics. Although not exhaustive (see Supplementary Table 1), this figure shows that both non-specific and antigen-specific therapies are being tested, which inhibit effector cells and antigen presentation as well as boost regulatory pathways. Purple and green dotted arrows indicate the therapeutics, black arrows are immune and metabolic pathways; a green dotted arrow indicates a positive effect and a purple dotted arrow indicates a negative effect. In addition, these immunotherapies are being combined with drugs that promote β-cell survival to potentially replenish insulin-producing β-cells. The figure has been redrawn after ref. , with permission.
Similar articles
- Immunotherapy in autoimmune type 1 diabetes.
Weigmann B, Franke RK, Daniel C. Weigmann B, et al. Rev Diabet Stud. 2012 Summer-Fall;9(2-3):68-81. doi: 10.1900/RDS.2012.9.68. Epub 2012 Nov 15. Rev Diabet Stud. 2012. PMID: 23403703 Free PMC article. Review. - Risk factors and primary prevention trials for type 1 diabetes.
Wu YL, Ding YP, Gao J, Tanaka Y, Zhang W. Wu YL, et al. Int J Biol Sci. 2013 Jul 18;9(7):666-79. doi: 10.7150/ijbs.6610. Print 2013. Int J Biol Sci. 2013. PMID: 23904791 Free PMC article. Review. - The pathogenesis, prediction, and prevention of insulin-dependent diabetes mellitus.
Muir A, Schatz DA, Maclaren NK. Muir A, et al. Endocrinol Metab Clin North Am. 1992 Jun;21(2):199-219. Endocrinol Metab Clin North Am. 1992. PMID: 1612064 Review. - New and future immunomodulatory therapy in type 1 diabetes.
Tooley JE, Waldron-Lynch F, Herold KC. Tooley JE, et al. Trends Mol Med. 2012 Mar;18(3):173-81. doi: 10.1016/j.molmed.2012.01.001. Epub 2012 Feb 17. Trends Mol Med. 2012. PMID: 22342807 Free PMC article. Review. - Diabetes research: are we getting anywhere?
Zinman B. Zinman B. CMAJ. 1996 Nov 1;155(9):1271-4. CMAJ. 1996. PMID: 8911293 Free PMC article.
Cited by
- Immunotherapy in autoimmune type 1 diabetes.
Weigmann B, Franke RK, Daniel C. Weigmann B, et al. Rev Diabet Stud. 2012 Summer-Fall;9(2-3):68-81. doi: 10.1900/RDS.2012.9.68. Epub 2012 Nov 15. Rev Diabet Stud. 2012. PMID: 23403703 Free PMC article. Review. - Reversal of New-Onset Type 1 Diabetes With an Agonistic TLR4/MD-2 Monoclonal Antibody.
Bednar KJ, Tsukamoto H, Kachapati K, Ohta S, Wu Y, Katz JD, Ascherman DP, Ridgway WM. Bednar KJ, et al. Diabetes. 2015 Oct;64(10):3614-26. doi: 10.2337/db14-1868. Epub 2015 Jun 30. Diabetes. 2015. PMID: 26130764 Free PMC article. - Treatment of type 1 and type 2 diabetes mellitus with insulin detemir, a long-acting insulin analog.
Young JR, McAdam-Marx C. Young JR, et al. Clin Med Insights Endocrinol Diabetes. 2010;3:65-80. doi: 10.4137/CMED.S5330. Epub 2010 Dec 5. Clin Med Insights Endocrinol Diabetes. 2010. PMID: 22879788 Free PMC article. - Increase in the Expression of Glucose Transporter 2 (GLUT2) on the Peripheral Blood Insulin-Producing Cells (PB-IPC) in Type 1 Diabetic Patients after Receiving Stem Cell Educator Therapy.
Zhao Y, Veysman B, Antolijao K, Zhao Y, Papagni Y, Wang H, Ross R, Tibbot T, Povrzenic D, Fox R. Zhao Y, et al. Int J Mol Sci. 2024 Jul 30;25(15):8337. doi: 10.3390/ijms25158337. Int J Mol Sci. 2024. PMID: 39125908 Free PMC article. - Gene CNVs and protein levels of complement C4A and C4B as novel biomarkers for partial disease remissions in new-onset type 1 diabetes patients.
Kingery SE, Wu YL, Zhou B, Hoffman RP, Yu CY. Kingery SE, et al. Pediatr Diabetes. 2012 Aug;13(5):408-18. doi: 10.1111/j.1399-5448.2011.00836.x. Epub 2011 Dec 13. Pediatr Diabetes. 2012. PMID: 22151770 Free PMC article.
References
- Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. - PubMed
- Vauzelle-Kervroedan F, et al. Analysis of mortality in French diabetic patients from death certificates: a comparative study. Diabete Metab. 1999;25:404–411. - PubMed
- Maahs DM, Rewers M. Editorial: mortality and renal disease in type 1 diabetes mellitus—progress made, more to be done. J Clin Endocrinol Metab. 2006;91:3757–3759. - PubMed
- Harjutsalo V, Sjoberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371:1777–1782. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical