MicroRNA-210 regulates mitochondrial free radical response to hypoxia and krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU - PubMed (original) (raw)
. 2010 Apr 26;5(4):e10345.
doi: 10.1371/journal.pone.0010345.
Anassuya Ramachandran, Robert McCormick, Harriet Gee, Christine Blancher, Meredith Crosby, Cecilia Devlin, Christopher Blick, Francesca Buffa, Ji-Liang Li, Borivoj Vojnovic, Ricardo Pires das Neves, Peter Glazer, Francisco Iborra, Mircea Ivan, Jiannis Ragoussis, Adrian L Harris
Affiliations
- PMID: 20436681
- PMCID: PMC2859946
- DOI: 10.1371/journal.pone.0010345
MicroRNA-210 regulates mitochondrial free radical response to hypoxia and krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU
Elena Favaro et al. PLoS One. 2010.
Abstract
Background: Hypoxia in cancers results in the upregulation of hypoxia inducible factor 1 (HIF-1) and a microRNA, hsa-miR-210 (miR-210) which is associated with a poor prognosis.
Methods and findings: In human cancer cell lines and tumours, we found that miR-210 targets the mitochondrial iron sulfur scaffold protein ISCU, required for assembly of iron-sulfur clusters, cofactors for key enzymes involved in the Krebs cycle, electron transport, and iron metabolism. Down regulation of ISCU was the major cause of induction of reactive oxygen species (ROS) in hypoxia. ISCU suppression reduced mitochondrial complex 1 activity and aconitase activity, caused a shift to glycolysis in normoxia and enhanced cell survival. Cancers with low ISCU had a worse prognosis.
Conclusions: Induction of these major hallmarks of cancer show that a single microRNA, miR-210, mediates a new mechanism of adaptation to hypoxia, by regulating mitochondrial function via iron-sulfur cluster metabolism and free radical generation.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
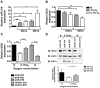
Figure 1. MiR-210 regulates ISCU expression in MCF7 cells.
A, miR-210 increases under hypoxia, with the most robust induction seen at 48 hrs with 0.1% oxygen. B, under the same conditions, the strongest downregulation of ISCU mRNA is observed at 48 hrs with 0.1% oxygen. The expression levels of miR-210 and ISCU mRNA under hypoxia are relative to their normoxic expression levels at 24 hrs (N). Mean ± s.e.m. of three independent experiments is shown (* p<0.05, ** p<0.01, *** p<0.001). C, transfection of anti-210 partially rescues the hypoxic suppression of ISCU mRNA and mimic-210 overexpression decreases ISCU mRNA in normoxia at 48 hrs. Expression of ISCU mRNA is relative to the anti-ctrl in normoxia (N). Mean ± s.e.m. of three independent experiments is shown (** p<0.01, *** p<0.001). D, (top panel) ISCU protein is downregulated in MCF7 lysates at 0.1% oxygen compared to normoxic lysates (N) when the anti-ctrl is transfected for 48 hrs. This decrease in protein level under hypoxic conditions is partially rescued when the anti-210 is transfected. Under normoxic conditions, mimic-210 suppresses ISCU protein levels compared to mimic-ctrl transfected cells. D, (bottom panel), quantification of the rescue of ISCU protein level upon transfection of anti-210. Transfection of anti-210 rescues the ISCU protein levels in MCF7 by approximately 40%. Mean ± S.E.M of two independent experiments is shown. (* p<0.05).
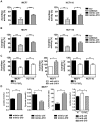
Figure 2. MiR-210 negatively regulates aconitase and complex I activity in MCF7 and HCT116, and upregulates glycolysis.
Cells transfected with siRNA against ISCU or with mimic-210 show a significant decrease in the activity of the Fe-S protein aconitase (A) and complex I (B) at 48 hrs. Activity is expressed relative to scr for siISCU3 or to mimic-ctrl for mimic-210. Mean ± s.e.m. of three independent experiments is shown (* p<0.05, ** p<0.01, *** p<0.001). C, hypoxia decreases aconitase and complex I activity in MCF7 and HCT116. Activity is expressed relative to normoxia (N). Transfection of MCF7 with anti-210 increases the level of aconitase activity, compared to anti-ctrl. Mean ± s.e.m. of three independent experiments is shown (* p<0.05, ** p<0.01, *** p<0.001). D, in normoxia, MCF7 transfected with mimic-210 have an increase in the secreted lactate concentration and a reduction in the extracellular pyruvate after 48 hours. Lactate: Pyruvate ratio shows a significant increase under the same conditions. Transfection with anti-210 in hypoxia reduced lactate production compared to anti-ctrl transfected cells. Mean ± s.e.m. of three biological replicates is shown (* p<0.05, ** p<0.01, *** p<0.001).
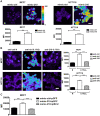
Figure 3. MiR-210 increases the formation of ROS in MCF7 and HCT116 cell lines.
A, in normoxia, transfection of mimic-210 significantly increases superoxide production at 48 hrs as measured by MitoSox staining. Representative images from mimic-210 transfected cells are shown on the top panels and the mean ± s.e.m. of approximately 300 cells are shown on the bottom panels (***, p<0.001). B, exposure of cells to 0.1% oxygen for 48 hrs enhances the production of superoxide; this effect is almost completely reversed upon transfection of anti-210. Representative images from miR-210 transfected are shown on the left panels and the mean ± s.e.m. of approximately 300 cells are shown on the right panels (***, p<0.001). C, ROS production induced by mimic-210 is inhibited in MCF7 cells by cotrasfection with the ISCU2 expressing plasmid. Mean ± s.e.m. of approximately 300 cells is shown (*** p<0.001). Bars = 10 µm.
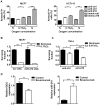
Figure 4. MiR-210 affects cell survival and is inversely correlated to ISCU in hypoxic tumor xenografts.
A, apoptosis (Annexin V+ cells) was measured in MCF7 (left) and HCT116 (right) cells treated with miR-210 inhibitor or mimic, after 48 hours exposure to 0.1% oxygen or normoxia. Mean ± s.e.m. is representative of 3 independent experiments (* p<0.05, ** p<0.01, *** p<0.001). B, after transfecting MCF7 cells with scrambled LNA or mir-210 LNA and exposed to 0.1% oxygen or normoxia (48 hrs), the cells were re-plated (100 and 500 cells/well). After a 12-day incubation, colonies were fixed, stained, and the surviving fractions were calculated based upon the plating efficiency. C, surviving fractions were calculated as in (B) in HeLa cells, which were transfected with anti-ctrl or anti-210 (Applied Biosystems/Ambion, Austin, TX, USA) and exposed to 0.01% oxygen or normoxia. In all colony assays, mean ± s.e.m. is representative of 2 independent experiments carried out in triplicate employing 2 different plating densities. (** p<0.01, *** p<0.001). D, RT-PCR for ISCU and miR-210 in U87 xenografts treated with anti-angiogenic therapy (bevacizumab). Relative expression of ISCU normalised to β-actin, miR-210 normalised to three small nucleolar controls, RNU43, RNU44 and RNU48. Mean expression ± s.e.m. in 4 animals/group is shown (** p<0.01, ***p<0.001).
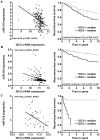
Figure 5. ISCU suppression is associated to miR-210 over-expression and relapse-free survival in breast cancer and HNSCC.
A, miR-210 qPCR expression (DDCT) plotted as a function of ISCU mRNA expression (Illumina arrays) in the Oxford breast cancer series (left), and, relapse free survival for samples with high ISCU and low ISCU split by median in the Oxford breast cancer series (right). B, mRNA expression of the transcript hosting the miR-210 precursor plotted as a function of ISCU mRNA expression measure by Affymetrix U133b and U133a respectively in the Uppsala breast cancer series (left), and relapse free survival for samples with high ISCU and low ISCU split by median in the Uppsala breast cancer series (right). C, miR-210 qPCR expression (DDCT) plotted as a function of ISCU mRNA expression (Affymetrix arrays) in the Oxford-Manchester HNSCC cancer series where ISCU was initially found to be associated to hypoxia (left), and relapse free survival for samples with high ISCU and low ISCU split by median value in the Chung et al. HNSCC series (right).
Similar articles
- MicroRNA-210 decreases heme levels by targeting ferrochelatase in cardiomyocytes.
Qiao A, Khechaduri A, Kannan Mutharasan R, Wu R, Nagpal V, Ardehali H. Qiao A, et al. J Am Heart Assoc. 2013 Apr 22;2(2):e000121. doi: 10.1161/JAHA.113.000121. J Am Heart Assoc. 2013. PMID: 23608607 Free PMC article. - Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression.
Chen Z, Li Y, Zhang H, Huang P, Luthra R. Chen Z, et al. Oncogene. 2010 Jul 29;29(30):4362-8. doi: 10.1038/onc.2010.193. Epub 2010 May 24. Oncogene. 2010. PMID: 20498629 - Clinically relevant HIF-1α-dependent metabolic reprogramming in oropharyngeal squamous cell carcinomas includes coordinated activation of CAIX and the miR-210/ISCU signaling axis, but not MCT1 and MCT4 upregulation.
Sáenz-de-Santa-María I, Bernardo-Castiñeira C, Secades P, Bernaldo-de-Quirós S, Rodrigo JP, Astudillo A, Chiara MD. Sáenz-de-Santa-María I, et al. Oncotarget. 2017 Feb 21;8(8):13730-13746. doi: 10.18632/oncotarget.14629. Oncotarget. 2017. PMID: 28099149 Free PMC article. - Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning.
Semenza GL. Semenza GL. Biochim Biophys Acta. 2011 Jul;1813(7):1263-8. doi: 10.1016/j.bbamcr.2010.08.006. Epub 2010 Aug 21. Biochim Biophys Acta. 2011. PMID: 20732359 Free PMC article. Review. - The role of hypoxia regulated microRNAs in cancer.
McCormick R, Buffa FM, Ragoussis J, Harris AL. McCormick R, et al. Curr Top Microbiol Immunol. 2010;345:47-70. doi: 10.1007/82_2010_76. Curr Top Microbiol Immunol. 2010. PMID: 20549470 Review.
Cited by
- MicroRNA-210 decreases heme levels by targeting ferrochelatase in cardiomyocytes.
Qiao A, Khechaduri A, Kannan Mutharasan R, Wu R, Nagpal V, Ardehali H. Qiao A, et al. J Am Heart Assoc. 2013 Apr 22;2(2):e000121. doi: 10.1161/JAHA.113.000121. J Am Heart Assoc. 2013. PMID: 23608607 Free PMC article. - MIR-210 modulates mitochondrial respiration in placenta with preeclampsia.
Muralimanoharan S, Maloyan A, Mele J, Guo C, Myatt LG, Myatt L. Muralimanoharan S, et al. Placenta. 2012 Oct;33(10):816-23. doi: 10.1016/j.placenta.2012.07.002. Epub 2012 Jul 26. Placenta. 2012. PMID: 22840297 Free PMC article. - Type of training has a significant influence on the GH/IGF-1 axis but not on regulating miRNAs.
Khalid K, Szewczyk A, Kiszałkiewicz J, Migdalska-Sęk M, Domańska-Senderowska D, Brzeziański M, Lulińska E, Jegier A, Brzeziańska-Lasota E. Khalid K, et al. Biol Sport. 2020 Sep;37(3):217-228. doi: 10.5114/biolsport.2020.94248. Epub 2020 May 23. Biol Sport. 2020. PMID: 32879543 Free PMC article. - Regulation of cellular sterol homeostasis by the oxygen responsive noncoding RNA lincNORS.
Wu X, Niculite CM, Preda MB, Rossi A, Tebaldi T, Butoi E, White MK, Tudoran OM, Petrusca DN, Jannasch AS, Bone WP, Zong X, Fang F, Burlacu A, Paulsen MT, Hancock BA, Sandusky GE, Mitra S, Fishel ML, Buechlein A, Ivan C, Oikonomopoulos S, Gorospe M, Mosley A, Radovich M, Davé UP, Ragoussis J, Nephew KP, Mari B, McIntyre A, Konig H, Ljungman M, Cousminer DL, Macchi P, Ivan M. Wu X, et al. Nat Commun. 2020 Sep 21;11(1):4755. doi: 10.1038/s41467-020-18411-x. Nat Commun. 2020. PMID: 32958772 Free PMC article. - Identification of a set of miRNAs differentially expressed in transiently TIA-depleted HeLa cells by genome-wide profiling.
Sánchez-Jiménez C, Carrascoso I, Barrero J, Izquierdo JM. Sánchez-Jiménez C, et al. BMC Mol Biol. 2013 Feb 6;14:4. doi: 10.1186/1471-2199-14-4. BMC Mol Biol. 2013. PMID: 23387986 Free PMC article.
References
- Harris AL. Hypoxia–a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. - PubMed
- Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE 2007. 2007:cm8. - PubMed
- Semenza GL. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J. 2007;405:1–9. - PubMed
- Bell EL, Chandel NS. Mitochondrial oxygen sensing: regulation of hypoxia-inducible factor by mitochondrial generated reactive oxygen species. Essays Biochem. 2007;43:17–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases