Tbx1 regulates Vegfr3 and is required for lymphatic vessel development - PubMed (original) (raw)
Tbx1 regulates Vegfr3 and is required for lymphatic vessel development
Li Chen et al. J Cell Biol. 2010.
Abstract
Lymphatic dysfunction causes several human diseases, and tumor lymphangiogenesis is implicated in cancer spreading. TBX1 is the major gene for DiGeorge syndrome, which is associated with multiple congenital anomalies. Mutation of Tbx1 in mice recapitulates the human disease phenotype. In this study, we use molecular, cellular, and genetic approaches to show, unexpectedly, that Tbx1 plays a critical role in lymphatic vessel development and regulates the expression of Vegfr3, a gene that is essential for lymphangiogenesis. Tbx1 activates Vegfr3 transcription in endothelial cells (ECs) by binding to an enhancer element in the Vegfr3 gene. Conditional deletion of Tbx1 in ECs causes widespread lymphangiogenesis defects in mouse embryos and perinatal death. Using the mesentery as a model tissue, we show that Tbx1 is not required for lymphatic EC differentiation; rather, it is required for the growth and maintenance of lymphatic vessels. Our findings reveal a novel pathway for the development of the lymphatic vessel network.
Figures
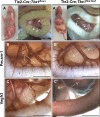
Figure 1.
Lymphatic abnormalities in EC-specific _Tbx1_-null mutants. (A) In control embryos, chyle is visible in mesenteric lymphatic vessels (arrowheads). (A’) On P4, Tie2-Cre; Tbx1flox/lacz mutants show growth retardation, abdominal distention, and accumulation of chyle in the intestinal wall (arrows) and membranous mesentery (arrowheads). (B and B′) Immunohistochemistry on isolated intestines at E18.5. Anti–Pecam-1 shows that Tie2Cre; Tbx1flox/lacz mutants (B′) have fewer mesenteric vessels than controls (B). (C′) Anti-Vegfr3 reveals that Tie2Cre/+; Tbx1flox/lacz mutants lack the mesenteric lymphatic vessels. (C) Control mesentery is shown. L, lymphatic vessel; A, artery; V, vein; I, intestine. Bars, 1 mm.
Figure 2.
Tbx1 colocalizes with LEC-specific markers in mesenteric lymphatic vessels. (A and B) LacZ reporter activity revealed by X-gal staining (blue) colocalizes with anti-Vegfr3 immunostaining (brown) in mesenteric lymphatic vessels (arrows) of Tbx1lacZ/+ embryos at E13.5. The boxed area in A is magnified in B. (C and D) Colocalization of LacZ reporter activity and anti-Lyve1 immunostaining in Tbx1lacZ/+ embryos at E14.5 (C) and E16.5 (D). (C, inset) X-gal staining on an adjacent section is shown. (E and F) Tbx1 protein is expressed in mesenteric LECs at E16.5 (E, arrowheads) and has a similar distribution to the LacZ reporter activity at this stage (F). A, mesenteric artery; V, mesenteric vein; L, lymphatic vessel. Bars, 100 µm.
Figure 3.
Fate mapping of _Tbx1_-expressing cells in the mesentery. (A and A′) X-gal staining of isolated intestines at E14.5 shows extensive contribution of _Tbx1_-traced cells to the mesenteric vessels of control (A; Tbx1Cre/+; R26R) and mutant (Tbx1Cre/ΔE5; R26R) embryos (A’). Black lines indicate the angle of sections in B and B′. (B and B′) In the proximal mesentery of E14.5 embryos, X-gal staining and anti-Lyve1 colocalize in mesenteric LECs in control (B) and mutant (B′) embryos. Note the abnormal anatomy of the mutant lymphatic vessels (B’, red arrowheads). (C and C’) At E15.5, in the proximal mesentery, highly abnormal lymphatic vessels in mutant embryos (C’, red arrowheads) are both X-gal+ and Lyve1+. (D and D’) In the distal mesentery, lymphatic vessels were present in control embryos (D, arrowhead) but not mutant embryos (D’). Insets show the boxed area at higher magnification. (E and E’) At E16.5, very few X-gal+/Lyve1+ vessels are identifiable (E’, red arrowhead). Note that the vasa vasorum (E′, black arrows) is X-gal+/Lyve1 negative. A, mesenteric artery; V, mesenteric vein; L, lymphatic vessel; P, proximal; D, distal. Black arrowheads indicate lymphatic vessels. Bars, 100 µm.
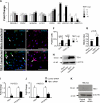
Figure 4.
Tbx1 activates prolymphangiogenic genes in ECs. (A) Fold change histogram representation of qRT-PCR results of RNA from HUVECs transfected with increasing amounts of TBX1 expression vector. (B–E) Anti-IGF1 (green) and anti-VEGFD (red) staining of _TBX1_-transfected HUVECs (D and E) and control cultures (B and C). Arrows indicate IGF1+ and VEGFD+ cells. Mock transfections (B, C, and F [white columns]) were performed by transfecting HUVECs with empty vector. TOPRO3 identifies cell nuclei. (F) More IGF1+ and VEGFD+ cells were present in _TBX1_-transfected cultures compared with control cultures. 500 cells were counted in three independent experiments for each antibody. (G) Cell proliferation in _TBX1_-transfected HUVECs increased >1.8-fold above that of control cultures. (H) Western blotting reveals increased TBX1 protein expression after TBX1 transfection of HUVECs. (I and J) TBX1 and VEGFR3 mRNA levels in _TBX1_-siRNA– treated or control siRNA–treated HUVECs (I) and HMLECs (J) were quantified by qRT-PCR and normalized to GAPDH. Control expression levels were set to 1. In both cell types, TBX1 and VEGFR3 mRNA levels were greatly reduced after TBX1 knockdown. Values are mean ± SEM. (K) Western blotting with antibodies to TBX1 and β-actin showed that TBX1 protein expression was reduced in HMLECs treated with _TBX1_-siRNA. Bars, 100 µm.
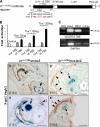
Figure 5.
Tbx1 regulates VEGFR3 expression via a conserved TBE in the endogenous VEGFR3 gene. (A) Schematic of the In11-12TBE luciferase reporter. A mutant version of the reporter construct was generated by site-directed mutagenesis of the TBE located within the putative VEGFR3 enhancer (In11-12TBE). (B) Cotransfection of JEG3 cells with the luciferase reporter and increasing amounts of Tbx1 expression vector increased activity of the luciferase reporter containing the wild-type enhancer (wt-TBE) but not the mutant enhancer (mut-TBE). Control cultures were transfected with empty expression vector. (C) PCR of chromatin immunoprecipitated from HUVECs. Antibodies used for immunoprecipitation are indicated above the panel. A chromatin sample immunoprecipitated with a nonspecific antibody (c-Myc) served as a negative control and the input sample as a positive control. Samples immunoprecipitated with anti-Tbx1 showed enrichment of the VEGFR3 enhancer containing the conserved TBE. (D–E′) Histological sections of the mesentery of transgenic embryos carrying the wild-type (D and E) or mutant TBE (D’ and E’) enhancer construct at E15.5. Colocalization of β-gal expression and anti-Vegfr3 immunostaining was seen in mesenteric LECs of transgenic embryos carrying the wild-type TBE (D and E, arrowheads) but not with the mutated TBE (D’ and E’). Arrows indicate the mesenteric artery (A). V, mesenteric vein; L, lymphatic vessel. Bars, 100 µm.
Similar articles
- A dual role for Tbx1 in cardiac lymphangiogenesis through genetic interaction with Vegfr3.
Martucciello S, Turturo MG, Bilio M, Cioffi S, Chen L, Baldini A, Illingworth E. Martucciello S, et al. FASEB J. 2020 Nov;34(11):15062-15079. doi: 10.1096/fj.201902202R. Epub 2020 Sep 20. FASEB J. 2020. PMID: 32951265 - Tbx1 regulates brain vascularization.
Cioffi S, Martucciello S, Fulcoli FG, Bilio M, Ferrentino R, Nusco E, Illingworth E. Cioffi S, et al. Hum Mol Genet. 2014 Jan 1;23(1):78-89. doi: 10.1093/hmg/ddt400. Epub 2013 Aug 14. Hum Mol Genet. 2014. PMID: 23945394 - H-, N- and Kras cooperatively regulate lymphatic vessel growth by modulating VEGFR3 expression in lymphatic endothelial cells in mice.
Ichise T, Yoshida N, Ichise H. Ichise T, et al. Development. 2010 Mar;137(6):1003-13. doi: 10.1242/dev.043489. Development. 2010. PMID: 20179099 - Tbx1: Transcriptional and Developmental Functions.
Baldini A, Fulcoli FG, Illingworth E. Baldini A, et al. Curr Top Dev Biol. 2017;122:223-243. doi: 10.1016/bs.ctdb.2016.08.002. Epub 2016 Sep 1. Curr Top Dev Biol. 2017. PMID: 28057265 Review. - [Molecular mechanisms of lymphatic development].
Kubo H. Kubo H. Seikagaku. 2004 Sep;76(9):1210-6. Seikagaku. 2004. PMID: 15524110 Review. Japanese. No abstract available.
Cited by
- Disable 2, A Versatile Tissue Matrix Multifunctional Scaffold Protein with Multifaceted Signaling: Unveiling Role in Breast Cancer for Therapeutic Revolution.
Shah NN, Dave BP, Shah KC, Shah DD, Maheshwari KG, Chorawala MR. Shah NN, et al. Cell Biochem Biophys. 2024 Jun;82(2):501-520. doi: 10.1007/s12013-024-01261-5. Epub 2024 Apr 9. Cell Biochem Biophys. 2024. PMID: 38594547 Review. - Single-cell analysis of lymphatic endothelial cell fate specification and differentiation during zebrafish development.
Grimm L, Mason E, Yu H, Dudczig S, Panara V, Chen T, Bower NI, Paterson S, Rondon Galeano M, Kobayashi S, Senabouth A, Lagendijk AK, Powell J, Smith KA, Okuda KS, Koltowska K, Hogan BM. Grimm L, et al. EMBO J. 2023 Jun 1;42(11):e112590. doi: 10.15252/embj.2022112590. Epub 2023 Mar 13. EMBO J. 2023. PMID: 36912146 Free PMC article. - Transcription factors regulating vasculogenesis and angiogenesis.
Payne S, Neal A, De Val S. Payne S, et al. Dev Dyn. 2024 Jan;253(1):28-58. doi: 10.1002/dvdy.575. Epub 2023 Mar 6. Dev Dyn. 2024. PMID: 36795082 Free PMC article. Review. - Involvement of an Aberrant Vascular System in Neurodevelopmental, Neuropsychiatric, and Neuro-Degenerative Diseases.
Ishihara K, Takata K, Mizutani KI. Ishihara K, et al. Life (Basel). 2023 Jan 12;13(1):221. doi: 10.3390/life13010221. Life (Basel). 2023. PMID: 36676170 Free PMC article. Review. - VEGFR3 modulates brain microvessel branching in a mouse model of 22q11.2 deletion syndrome.
Cioffi S, Flore G, Martucciello S, Bilio M, Turturo MG, Illingworth E. Cioffi S, et al. Life Sci Alliance. 2022 Oct 10;5(12):e202101308. doi: 10.26508/lsa.202101308. Life Sci Alliance. 2022. PMID: 36216515 Free PMC article.
References
- D’Antonio L.D., Marsh J.L. 1987. Abnormal carotid arteries in the velocardiofacial syndrome. Plast. Reconstr. Surg. 80:471–472 - PubMed
- Emanuel B.S., McDonald-McGinn D., Saitta S.C., Zackai E.H. 2001. The 22q11.2 deletion syndrome. Adv. Pediatr. 48:39–73 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous