Cell wall synthesis is necessary for membrane dynamics during sporulation of Bacillus subtilis - PubMed (original) (raw)
Cell wall synthesis is necessary for membrane dynamics during sporulation of Bacillus subtilis
Pablo Meyer et al. Mol Microbiol. 2010 May.
Abstract
During Bacillus subtilis sporulation, an endocytic-like process called engulfment results in one cell being entirely encased in the cytoplasm of another cell. The driving force underlying this process of membrane movement has remained unclear, although components of the machinery have been characterized. Here we provide evidence that synthesis of peptidoglycan, the rigid, strength bearing extracellular polymer of bacteria, is a key part of the missing force-generating mechanism for engulfment. We observed that sites of peptidoglycan synthesis initially coincide with the engulfing membrane and later with the site of engulfment membrane fission. Furthermore, compounds that block muropeptide synthesis or polymerization prevented membrane migration in cells lacking a component of the engulfment machinery (SpoIIQ), and blocked the membrane fission event at the completion of engulfment in all cells. In addition, these compounds inhibited bulge and vesicle formation that occur in spoIID mutant cells unable to initiate engulfment, as did genetic ablation of a protein that polymerizes muropeptides. This is the first report to our knowledge that peptidoglycan synthesis is necessary for membrane movements in bacterial cells and has implications for the mechanism of force generation during cytokinesis.
Figures
Fig. 1
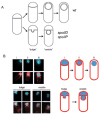
Membrane bulge and vesicle formation in engulfment mutants. A. Sporulating wild-type cells undergo engulfment where the smaller forespore compartment becomes a free cell within the larger mother cell (top). Strains carrying spoIID or spoIIP mutations are blocked at the stage of asymmetric septation and undergo membrane bulging, eventually leading to the formation of membrane bound vesicles (bottom). B. Wild-type (AES574) and spoIID strains (JDB2494) expressing CFP (blue) under control of a forespore-specific promoter (P_spoIIQ_) as a forespore marker were imaged at T4 in resuspension medium and stained with FM4-64 (red) to visualize membranes. Top, CFP is expressed in: (i) the forespore of wild-type cells undergoing asymmetric septation stained with FM4-64, (ii) engulfing cells stained with FM4-64 and (iii) engulfed cells not stained with FM4-64. Bottom, spoIID cells contained either a continuous CFP signal distribution between the forespore and the membrane protrusion (‘bulge’), or two distinguishable CFP signals separated by a membrane (‘vesicle’).
Fig. 2
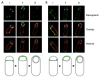
Peptidoglycan synthesis during engulfment. Engulfing cells contain sites of active peptidoglycan synthesis. Ramoplanin-FL (top) and FM4-64 (bottom) staining of (A) wild-type (PY79) cells and (B) spoIID(D210A) cells; centre panel are the overlay of the two images. (i) Ramoplanin-FL staining at septal tips, (ii) ramoplanin-FL staining around the forespore, (iii) ramoplanin-FL staining restricted to the point of contact between the advancing arms. Diagrams shown represent ramoplanin-FL (green) signals at different stages of engulfment corresponding to wild-type (left) and spoIID(D210A) (right) images.
Fig. 3
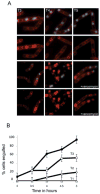
Inhibition of muropeptide synthesis blocks membrane migration in a spoIIQ mutant. All cultures were grown on agarose pads composed of A+B medium at 30°C and stained with FM4-64. The initial image in each sequence was taken at approximately T2 after sporulation initiation and arbitrarily set to t = 0 min. The time of subsequent images of the membrane stain is indicated in minutes in the lower right corner. A. Wild type (PY79). B. Wild type + 5 mM fosfomycin added at T1.5 after initiation of sporulation. C. spoIIQ (KP575). D. spoIIQ + 5 mM fosfomycin added at T1.5. E. Histogram showing the percentage of cells where membrane migrated in Δ_spoIIQ_ cells with (17 out of 55 cells or 75 ± 5%) or without (54 out of 72 cells or 30 ± 6%) 5 mM fosfomycin added at T1.5 after initiation of sporulation. Means are significantly different by _t_-test (P < 10−5). For complete movies, see Fig. S3. Scale bar = 1 μm.
Fig. 4
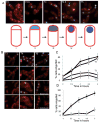
Inhibition of muropeptide synthesis blocks membrane fission. A. Assay of membrane fission. Top, sporulating wild-type cells (AES574) express CFP (blue) under control of a forespore specific promoter (P_spoIIQ_). Membranes of cells at intermediate stages of engulfment (T3–T4.5, green arrow) are also stained by a membrane impermeant dye (FM4-64, red) just before imaging; upon completion of membrane fission, cells are no longer stained by this dye (yellow arrow). Time after resuspension is indicated. Bottom, cells at different stages of sporulation. Prior to the completion of asymmetric septation (i), no CFP (blue) signal is observed. Following completion of asymmetric septation, a faint CFP signal is observed in the forespore (ii). As the cells proceed through engulfment (iii), the CFP signal increases. When membrane movement is complete (iv), the forespore remains attached to the mother cell and stained with FM4-64 (red). When the forespore is released from the mother cell (v), the CFP signal is no longer surrounded by an FM4-64 signal. B. Fosfomycin blocks engulfment before membrane separation. Sporulating wild-type cells were either treated with fosfomycin (5 mM) at T2 (bottom panels) or not treated (top panels). Time after resuspension is indicated. C. Inhibition of membrane fission by fosfomycin is dependent on time of addition. Wild-type cells (AES574) were untreated (filled circles) or fosfomycin (5 mM) was added at T1 (triangles), T2 (diamonds) or T3 (open circles) and the fraction of cells with a CFP signal not surrounded by an FM4-64 signal was determined. D. Fosfomycin-resistant murAA mutant engulfs normally in the presence of fosfomycin. The effect of fosfomycin on engulfment of a merodiploid strain (JDB2426) expressing an IPTG-inducible C117D murAA allele and YFP under a forespore-specific promoter (P_spoIIQ_-yfp) was determined. Filled circles, both 1 mM IPTG was added at T0 and 5 mM fosfomycin was added at T2; open circles, only 5 mM fosfomycin at T2.
Fig. 5
Inhibition of muropeptide transpeptidation blocks completion of engulfment. A. Vancomycin blocks engulfment before membrane fission. At T2 of sporulation, cells (AES574) expressing CFP (blue) under control of a forespore-specific promoter (P_spoIIQ_) were either treated with vancomycin (0.5 μg ml−1; bottom rows) or not treated (top rows). Membranes were visualized with FM4-64 (red). Time after resuspension is indicated. B. Inhibition of membrane fission depends on the time of addition. The percentage of sporulating cells completing membrane fission was determined for untreated cells (filled circle) or cells incubated with vancomycin (0.5 μg ml−1) at T1 (triangles), T2 (diamonds) or T3.5 (open circles) of sporulation by resuspension.
Fig. 6
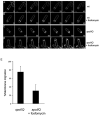
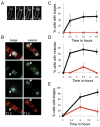
Muropeptide synthesis and polymerization are required for bulge and vesicle formation. A. Time-lapse images of a bulge progressively forming in a spoIID mutant strain (JDB2395) sporulating in a pad at 30°C and stained with FM4-64. Initial image was taken at approximately T2 after sporulation initiation and arbitrarily set to t = 0 min. The time of subsequent images is indicated in minutes in the lower right corner. See movie Fig. S7. B. In a spoIID mutant strain (JDB2494) expressing CFP (blue) in the forespore, bulge and vesicles (white arrows) are sites of peptidoglycan synthesis. Membranes were visualized with FM4-64 (red) and newly synthesized peptidoglycan identified by ramoplanin-FL, a fluorescent ramoplanin derivative (green). Time after start of sporulation is indicated. C. Fosfomycin blocks bulge formation. Bulges do not form in a strain (JDB2494) lacking spoIID when 5 mM fosfomycin was added at T2 of sporulation (red line) compared with cells where no fosfomycin was added (black line). D. Fosfomycin blocks vesicle formation. Percentage of cells with vesicles in strains carrying mutations in spoIID and expressing CFP at the forespore (JDB2494) in the absence (black) or in the presence of 5 mM fosfomycin added at T2 of sporulation (red). E. Vancomycin blocks bulge formation. Percentage of spoIID cells (JDB2494) with bulges in the absence (black) or in the presence of 0.5 μg ml−1 vancomycin (red) added at T2 of sporulation. Bulges and vesicles were identified as defined in Fig. 1B.
Fig. 7
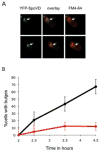
Muropeptide-polymerizing enzyme is required for bulge formation. A. The transpeptidase SpoVD localizes to the bulges. A strain lacking spoIIP and expressing a YFP–SpoVD fusion (JDB2553) was sporulated by resuspension and images acquired at T4. Two cells are shown. Left, YFP–SpoVD signal; middle is overlay of the YFP–SpoVD and FM4-64 signals; right, FM4-64. White arrows show the bulge. B. Bulge formation is suppressed by a spoVD mutation. Percentage of spoIIP (JDB2396; black) and spoIIP spoVD (JDB2537; red) cells with bulges was determined. Bulge formation was assessed as defined in Fig. 1B.
Fig. 8
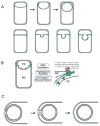
Role of peptidoglycan polymerization in engulfment. A. Diagram shows distribution of active peptidoglycan synthesis (green) during membrane migration and before detachment of the forespore from the mother cell (top) as well as during bulge formation (bottom). B. The polymerization of these newly synthesized muropeptides partially drives membrane movement and results in the separation of the two cells. Our data suggest that membrane movement can be mediated either by peptidoglycan synthesis (PG; green) or by the SpoIIQ–SpoIIIAH proteins (blue) and that peptidoglycan hydrolysis mediated by the DMP proteins (red) plays a necessary role in detaching the septal peptidoglycan from the transverse peptidoglycan. FS, forespore; MC, mother cell. C. Polymerization of newly synthesized muropeptides into peptidoglycan (green) at the point of contact between the migrating arms of the membranes pushes the septal membranes apart. Following detachment, the forespore becomes an independent membrane-bounded compartment in the cytoplasm. This polymerization is mediated by an unidentified transpeptidase-transglycosylase (red).
Similar articles
- Shaping an Endospore: Architectural Transformations During Bacillus subtilis Sporulation.
Khanna K, Lopez-Garrido J, Pogliano K. Khanna K, et al. Annu Rev Microbiol. 2020 Sep 8;74:361-386. doi: 10.1146/annurev-micro-022520-074650. Epub 2020 Jul 13. Annu Rev Microbiol. 2020. PMID: 32660383 Free PMC article. Review. - SpoIIQ-dependent localization of SpoIIE contributes to septal stability and compartmentalization during the engulfment stage of Bacillus subtilis sporulation.
Dehghani B, Rodrigues CDA. Dehghani B, et al. J Bacteriol. 2024 Jul 25;206(7):e0022024. doi: 10.1128/jb.00220-24. Epub 2024 Jun 21. J Bacteriol. 2024. PMID: 38904397 Free PMC article. - Genetic Screens Identify Additional Genes Implicated in Envelope Remodeling during the Engulfment Stage of Bacillus subtilis Sporulation.
Chan H, Taib N, Gilmore MC, Mohamed AMT, Hanna K, Luhur J, Nguyen H, Hafiz E, Cava F, Gribaldo S, Rudner D, Rodrigues CDA. Chan H, et al. mBio. 2022 Oct 26;13(5):e0173222. doi: 10.1128/mbio.01732-22. Epub 2022 Sep 6. mBio. 2022. PMID: 36066101 Free PMC article. - SpoIID-mediated peptidoglycan degradation is required throughout engulfment during Bacillus subtilis sporulation.
Gutierrez J, Smith R, Pogliano K. Gutierrez J, et al. J Bacteriol. 2010 Jun;192(12):3174-86. doi: 10.1128/JB.00127-10. Epub 2010 Apr 9. J Bacteriol. 2010. PMID: 20382772 Free PMC article. - Spore Peptidoglycan.
Popham DL, Bernhards CB. Popham DL, et al. Microbiol Spectr. 2015 Dec;3(6). doi: 10.1128/microbiolspec.TBS-0005-2012. Microbiol Spectr. 2015. PMID: 27337277 Review.
Cited by
- Mechanical consequences of cell-wall turnover in the elongation of a Gram-positive bacterium.
Misra G, Rojas ER, Gopinathan A, Huang KC. Misra G, et al. Biophys J. 2013 Jun 4;104(11):2342-52. doi: 10.1016/j.bpj.2013.04.047. Biophys J. 2013. PMID: 23746506 Free PMC article. - Small proteins link coat and cortex assembly during sporulation in Bacillus subtilis.
Ebmeier SE, Tan IS, Clapham KR, Ramamurthi KS. Ebmeier SE, et al. Mol Microbiol. 2012 May;84(4):682-96. doi: 10.1111/j.1365-2958.2012.08052.x. Epub 2012 Apr 18. Mol Microbiol. 2012. PMID: 22463703 Free PMC article. - The engulfasome in C. difficile: Variations on protein machineries.
Kelly A, Salgado PS. Kelly A, et al. Anaerobe. 2019 Dec;60:102091. doi: 10.1016/j.anaerobe.2019.102091. Epub 2019 Aug 27. Anaerobe. 2019. PMID: 31470088 Free PMC article. - Regulation of Clostridium difficile Spore Formation by the SpoIIQ and SpoIIIA Proteins.
Fimlaid KA, Jensen O, Donnelly ML, Siegrist MS, Shen A. Fimlaid KA, et al. PLoS Genet. 2015 Oct 14;11(10):e1005562. doi: 10.1371/journal.pgen.1005562. eCollection 2015 Oct. PLoS Genet. 2015. PMID: 26465937 Free PMC article. - Shaping an Endospore: Architectural Transformations During Bacillus subtilis Sporulation.
Khanna K, Lopez-Garrido J, Pogliano K. Khanna K, et al. Annu Rev Microbiol. 2020 Sep 8;74:361-386. doi: 10.1146/annurev-micro-022520-074650. Epub 2020 Jul 13. Annu Rev Microbiol. 2020. PMID: 32660383 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM081368/GM/NIGMS NIH HHS/United States
- R01 R01GM57045/GM/NIGMS NIH HHS/United States
- R01 GM081368-03/GM/NIGMS NIH HHS/United States
- R01GM83468/GM/NIGMS NIH HHS/United States
- R01 GM081368-01A2/GM/NIGMS NIH HHS/United States
- R01 GM057045/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases